What is pH?
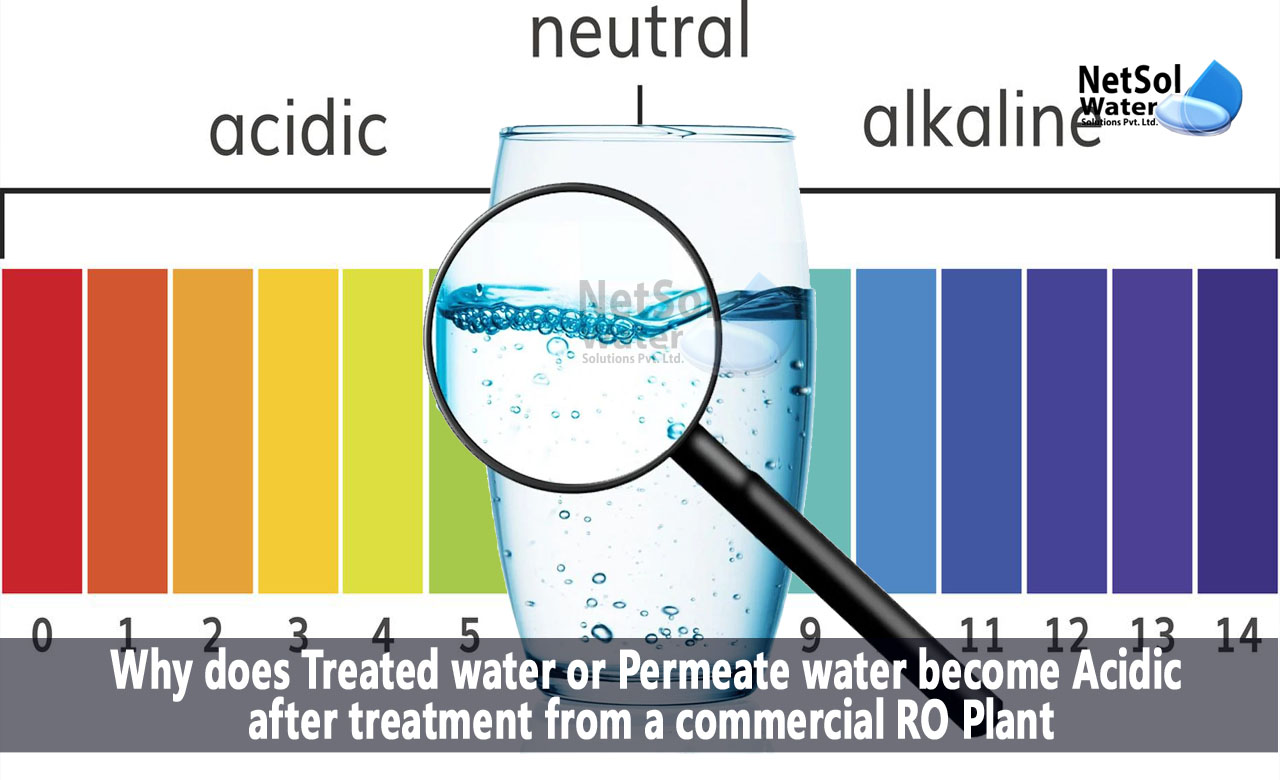
Hydrogen ion concentration in a solution is measured by pH. Any solution's pH is determined by the equilibrium between hydrogen (H+) and hydroxyl (OH) ions. Bases are those chemicals that result in an excess of (OH), whereas acids are those that result in an excess of H+ in solution. Equilibrium is the condition in which there are equal amounts of both in a pH 7 solution (neutral). The pH scale is logarithmic, and this is very important to remember. On the pH scale, there is a 10x difference between any two adjacent integers. Therefore, there is a 100X difference in the concentration of hydrogen ions between a pH of 4 and a pH of 6!
What is acidity?
Acidity is the term used to describe how acidic or basic a solution is, usually as determined by its pH. A pH of 7 is neutral, whereas a pH of 7 or less is acidic and a pH of 7 or more is basic. The pH scale has a range of 0 to 14, with 0 representing the most acidic and 14 representing the most basic. The amount of hydrogen ions (H+) a solution contains determines how acidic it is. While a solution with a low concentration of H+ ions is thought to be basic, one with a high concentration of H+ ions is seen to be acidic.
Why is RO water become acidic?
Through filtering, reverse osmosis, also known as RO, eliminates a significant amount of contaminants from water. This procedure results in water that is nearly pure and has a pH level of 7.
The pH level of reverse osmosis product water decreases to an acidic range of 6-6.5 when exposed to air, though. This happens as a result of pure water's propensity to absorb CO2 from the atmosphere, which can result in the production of carbonic acid (H2CO3) and a rapid drop in pH.
CO2 + H2O —-> H2CO3 (Carbonic Acid)
Reverse osmosis water does not have an alkaline pH since alkaline water is defined as having a pH over 7.
How to Check the pH of RO Water?
1. pH test strips: One of the cheapest and simplest ways to check the pH of RO water is with pH test strips. These reusable, low-cost plastic strips change colour after being soaked in water to provide a pH reading.
When measuring RO water, more exact pH strips are available that will provide an accurate reading for slightly acidic and slightly basic.
2. Litmus paper: Litmus paper is sort of a dumb method of testing pH but it does work. It will just tell you which type of water it is; it won't tell you how much of either an acid or a base it is. You can determine if you have an acid or a base by soaking litmus paper in water, which will cause it to change to one of two colors A digital pH meter is an additional choice for determining pH levels. Although it will provide the most accurate pH reading of any technique, it can be excessive if you only intend to test the water once.
3. A digital pH meter: A digital pH meter is an additional choice for determining pH levels. Although it will provide the most accurate pH reading of any technique, it can be excessive if you only intend to test the water once.
Conclusion:
In conclusion, water produced by the reverse osmosis method is not acidic. Instead, the pH level of the purified water made by reverse osmosis ranges from neutral to slightly alkaline. This is because contaminants and minerals that could make the water acidic are removed during the filtration process. Reverse osmosis water is an excellent option for individuals looking for clean and hygienic drinking water because it is also devoid of chemicals and other impurities.
Do you need an advice or assistance on selecting the best water and waste water treatment unit? We have solutions for all your problems!
Let us now your problem, our experts will make sure that it goes away.
For an assistance or related query,
Call on +91-965-060-8473
Or write us at enquiry@netsolwater.com