Introduction
Compounds react in a certain ratio in a chemical reaction. There will be a residual reactant if the ratio is uneven. To grasp this, you must be familiar with the molar or mole ratio.
What exactly do you mean by molar ratio?
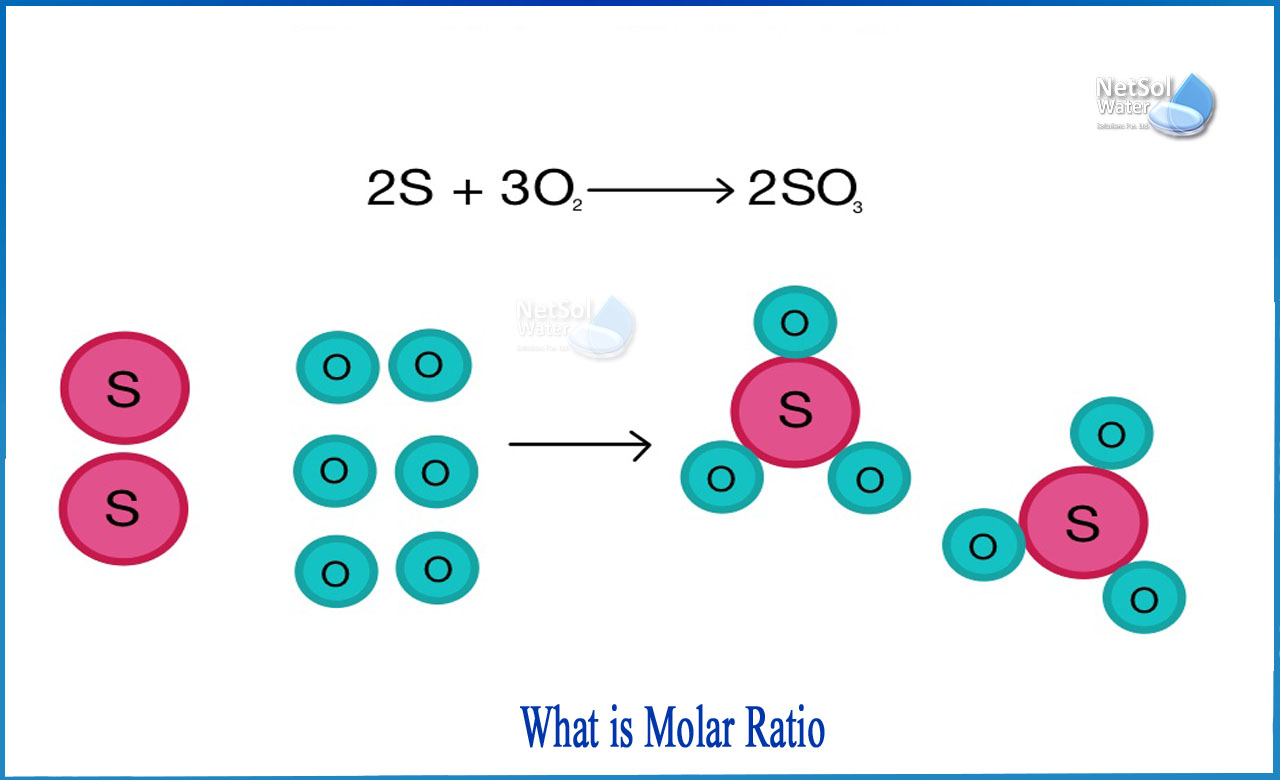
A mole ratio is the ratio of the mole quantities of any two compounds in a chemical process. In many chemical situations, mole ratios are employed as conversion factors between products and reactants. Examining the coefficients in front of formulae in a balanced chemical equation, can provide the mole ratio.
The significance of molar ratio
Chemical equations are representations of chemical processes in symbolic form.The reacting components are written on the left side of a chemical equation, and the products are written on the right; the two sides are generally divided by an arrow indicating the direction of the reaction. The absolute stoichiometric quantity employed in the reaction is denoted by the number coefficient next to each entity. Because the rule of conservation of mass states that the amount of each element must remain constant during a chemical reaction, each side of a balanced chemical equation must have the same amount of each element.
The coefficients in a balanced chemical equation can be used to calculate the relative quantity of molecules, formula units, or moles of chemicals involved in the reaction. The coefficients in a balanced equation may be used to calculate molar ratios, which can then be utilized as conversion factors to connect the reactants to the products. These conversion factors state the ratio of reactants participating in the reaction but do not specify how much of each component is really engaged in the process.
Units of Molar Ratio
Molar ratio units are either mole: mole or a dimensionless number since the units cancel each other out. For example, it is acceptable to state that a 3:1 ratio of 3 moles of O2 to 1 mole of H2 is correct, or it can also be stated as 3 mol O2: 1 mol H2.
Example of a Mole Ratio: A Balanced Equation
In response to the reaction:
2 H2(g) + O2(g) → 2 H2O(g)
The mole ratio of O2 to H2O is 1:2. For every mole of O2 consumed, two moles of H2O are produced.
H2 and H2O have a mole ratio of 1:1.
2 moles of H2O are generated for every 2 moles of H2. If four moles of hydrogen are utilized, four moles of water are generated.
Example of an Unbalanced Equation
Let's take another imbalanced equation as an example:
O3 → O2
This equation is not balanced since mass is not conserved, as may be seen by inspection. Ozone (O3) has more oxygen atoms than oxygen gas (O2). A mole ratio cannot be calculated for an imbalanced equation.
When this equation is balanced, the following results:
2O3 → 3O2
You may now calculate the mole ratio by plugging the coefficients in front of ozone and oxygen.
The ozone-to-oxygen ratio is 2:3. How will you put this to use?
Assume you are requested to calculate how many grams of oxygen are created when 0.2 grams of ozone are reacted!
The first step is to determine how many moles of ozone are contained in 0.2 grams. (Because it's a molar ratio, the ratio for grams isn't the same in most calculations.)
Look up the atomic weight of oxygen in the periodic table to convert grams to moles. Per mole, there are 16.00 grams of oxygen.
To determine how many moles are in 0.2 grams, solve for:
X moles = 0.2 g * (1 mole/16.00 g)
You will receive 0.0125 moles
Calculate how many moles of oxygen are created by 0.0125 moles of ozone using the mole ratio:
* (3 moles oxygen/2 moles ozone) moles of oxygen = 0.0125 moles ozone
When you solve this, you obtain 0.01875 moles of oxygen gas
Finally, for the solution, convert the amount of moles of oxygen gas into grams:
0.01875 moles * (16.00 grams/mole) = grams of oxygen gas
0.3 gram = 0.3 gram of oxygen gas
Because just one sort of atom was present on both sides of the equation, it should be very evident that you could have put the mole fraction straight away in this specific example. However, knowing the technique is useful for, when you encounter more difficulties to tackle.
Netsol Water is Greater Noida-based leading water & wastewater treatment plant manufacturer. We are industry's most demanding company based on client review and work quality. We are known as best commercial RO plant manufacturers, industrial RO plant manufacturer, sewage treatment plant manufacturer, Water Softener Plant Manufacturers and effluent treatment plant manufacturers. Apart from this 24x7 customer support is our USP. Call on +91-9650608473, or write us at enquiry@netsolwater.com for any support, inquiry or product-purchase related query.