How to Remove Suspended Particles Using Coagulation and Flocculation?
Coagulation and flocculation are important processes used in water and wastewater treatment plants to remove suspended particles from water. They are often used together to effectively remove particulates that would otherwise cause the water to be turbid or colored. We explain what coagulation and flocculation are, how they work, and the typical steps involved in using them to clarify water.
What is Coagulation?
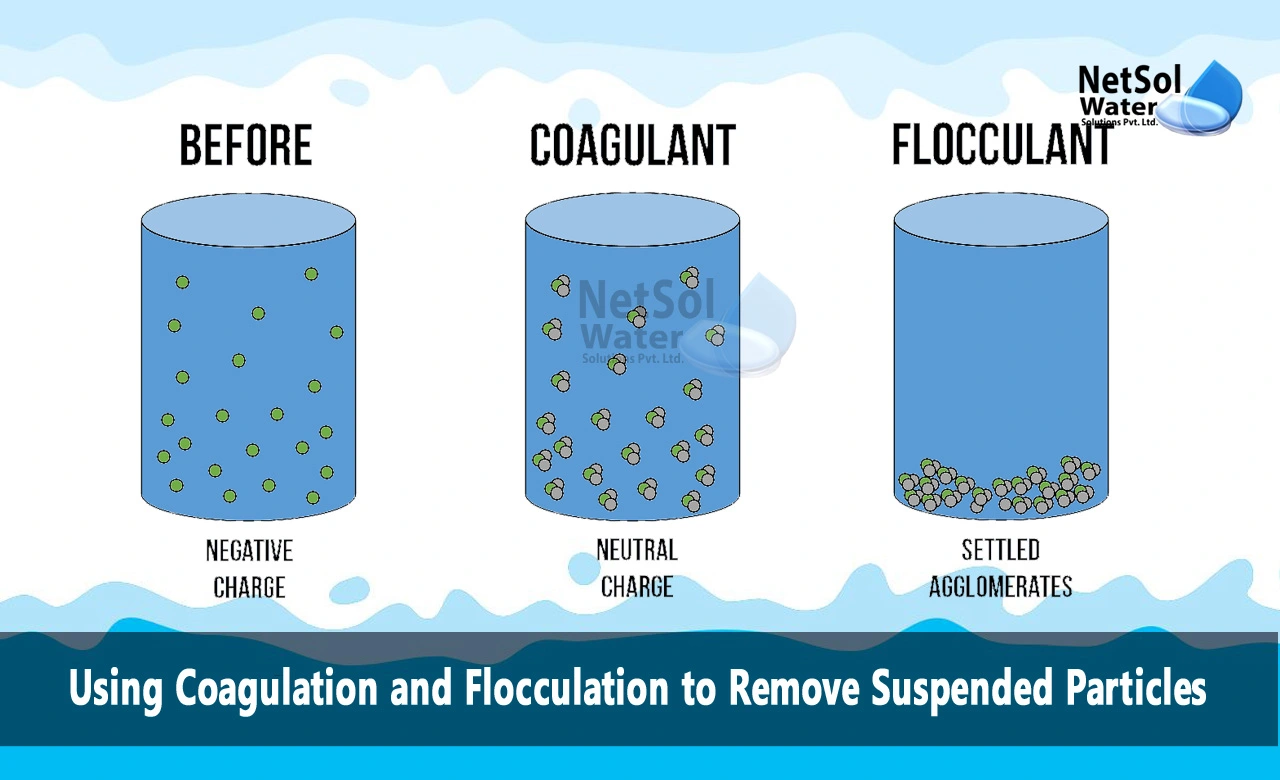
Coagulation is the process of destabilizing the charges on particles suspended in water, causing them to clump together and form larger flocs. It involves adding chemicals called coagulants to the water, which neutralize the negative electrical surface charges on the suspended particles. Common coagulants used include aluminum sulfate, ferric chloride, and ferric sulfate.
When the coagulant is added, the metal ions hydrolyze and react with the water to form insoluble metal hydroxide precipitates. These positively charged precipitates neutralize the negative surface charges on the suspended particles, reducing the repulsive forces between them. This allows the particles to collide, coagulate, and aggregate together into larger clumps or flocs.
What is Flocculation?
Flocculation follows coagulation and helps promote the aggregation of the destabilized particles so they can be separated from the water. It is the process of gentle mixing after the coagulant has been added, which causes dispersed particles to collide, aggregate and form larger flocs.
Long-chain polymer flocculants may also be added to strengthen the flocs and create larger agglomerations of particles. The floc particles are brought into contact through the process of gentle mixing by either mechanical or hydraulic means. This mixing helps accelerate the rate of contact of colloidal particles so they can aggregate and form flocs.
Typical Coagulation and Flocculation Process
The typical process for coagulation and flocculation in a water treatment plant involves the following steps:
1. Coagulant dosing - The coagulant (e.g. aluminum sulfate) is added and rapidly mixed with the water. This allows dispersion of the coagulant and immediate particle destabilization.
2. Flash mixing - The water is quickly mixed to promote coagulant dispersion and uniform particle destabilization. This rapid mixing also distributes the coagulant evenly for consistent treatment.
3. Flocculation - The water then goes through a period of gentle but constant mixing for flocculation. The floc particles collide, aggregate, and grow in size during this slow mixing process.
4. Sedimentation - The water flows into sedimentation basins where the heavy flocs settle out by gravity and are removed.
5. Filtration - The clarified water often undergoes additional granular media filtration to capture any remaining flocs and particulates.
6. pH correction - Acids or bases may need to be added after coagulation to readjust the final pH for distribution.
Conclusion
Coagulation and Flocculation using chemicals like aluminum sulfate is an important treatment process used to aggregate suspended particles in water into larger flocs for effective removal. Coagulation destabilizes the particle charges while flocculation causes collisions and aggregation through gentle mixing. With proper implementation, these processes can clarify turbid water by generating floc formations that settle out, producing a cleaner effluent for supply.
Do you need an advice or assistance on selecting the best water and wastewater treatment unit? We have solutions for all your problems!
Let us know your problem, our experts will make sure that it goes away.
For an assistance or related query,
Call on +91-965-060-8473 Or write us at enquiry@netsolwater.com