Overview
Mechanical filtration is commonly used to remove impurities from drinking water. But what if there are dissolved or too small particles in the water that cannot be removed by mechanical filtration?
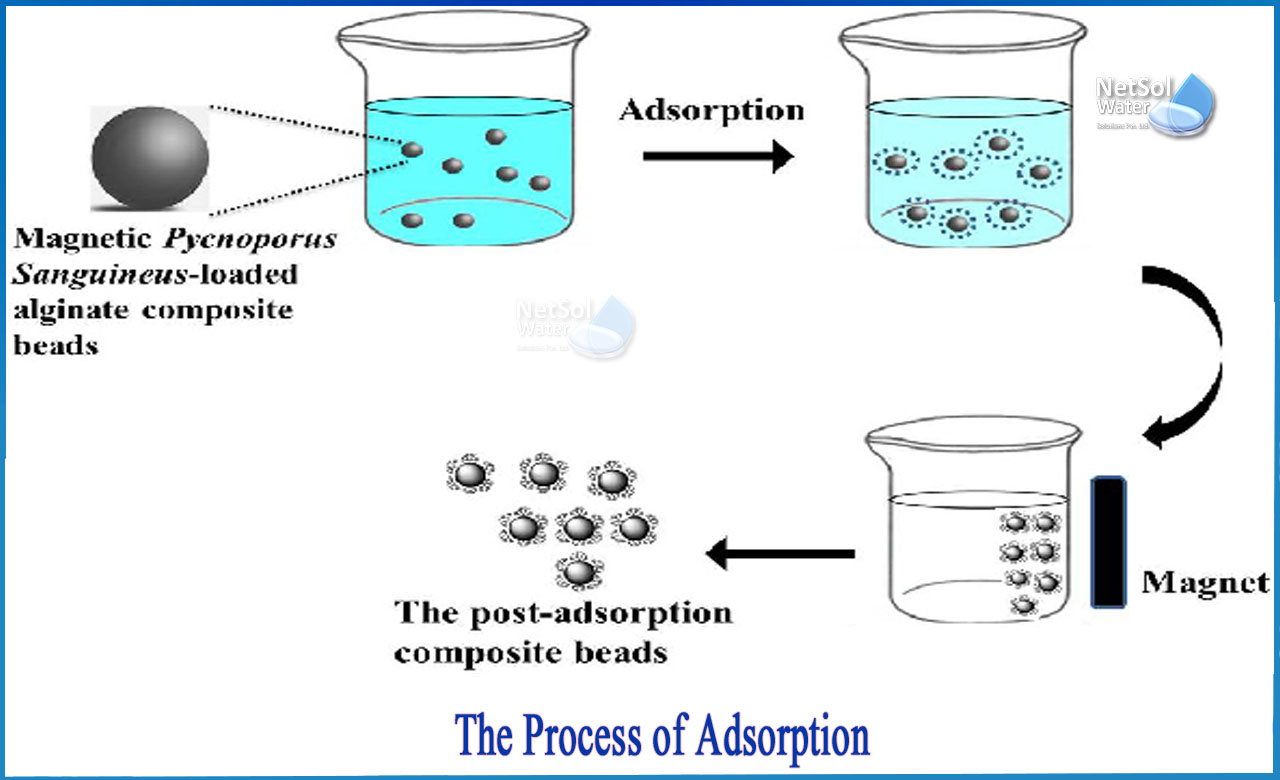
Adsorption, fortunately, is a process that can remove very small particles or dissolved contaminants from water, such as lead, PCBs, some pesticides, viruses, and asbestos fibers.
What is adsorption?
Adsorption is a process that is widely used in water treatment. The term refers to the adhesion of a thin layer of molecules to the surfaces of liquids or solids with which they come into contact.
Adsorption is a surface process that causes a molecule to move from a fluid bulk to a solid surface. This can happen as a result of physical forces or chemical bonds. When it is reversible (the opposite process is called desorption), it is responsible not only for substance subtraction but also for substance release.
In the majority of cases, this process is described at equilibrium by some equations that quantify the amount of substance attached to the surface given the concentration in the fluid. These equations are known as isotherms (the most famous of which are the Langmuir and Freundlich equations) because their parameters depend on temperature, which is one of the most important environmental factors affecting adsorption.
Why is the process of adsorption used?
Adsorption is widely used to remove organic substances from drinking water, in tertiary wastewater treatment, and in groundwater remediation. It is also used in home water treatment as well as in the treatment of water in aquariums and swimming pools.
The adsorption principle and the ability of certain solid materials to remove dissolved substances from water have long been understood. Adsorption technology has been used to a greater extent for water treatment for about 100 years, and it has not lost its relevance during this time. On the contrary, new application fields, such as groundwater remediation or enhanced wastewater treatment, have been added in recent decades, in addition to the traditional application in drinking water treatment.
Adsorptive Substances
This water treatment process can employ a wide range of solid materials, including flocculants, substances such as aluminum oxide or iron hydroxide, and synthetic materials. These materials have sites, such as tiny pores, where unwanted substances can cling. Activated carbon is a common type of adsorptive material.
The large number of pores in activated carbon allows it to attract and retain unwanted compounds. These provide activated carbon with a specific surface area of 500 to 1,500 square meters per gramme.
In 1930, activated carbon was used for the first time in municipal water treatment. Activated carbon is still a widely used adsorption treatment. It has the ability to purify, deodorize, and dechlorinate water. Some heavy metals, tannins, and volatile organic compounds can also be removed by activated carbon filters.
How is activated carbon created?
Activated carbon is created by heating carbon-based materials such as coal or wood without oxygen to produce charcoal. The charred material is then heated with steam or carbon dioxide to temperatures above 1000 degrees Celsius, further eroding and corroding it to remove all but the carbon.
The end result is an airy, delicate structure made almost entirely of carbon and riddled with holes. It is then crushed to a powder and combined with binders to form granules of various sizes for various filter media.
Chemicals are sometimes added to the carbon during activation to create different surface chemical natures that adsorb different contaminants. Acids, for example, generate carbon with a high capacity for heavy metal adsorption.
The enormous surface area of activated carbon is a critical factor in its ability to adsorb various contaminants. The surface area per gramme is typically around 1,000 square meters. A pea-sized piece of carbon, for example, has the area of half a football field. Because the size of molecules that can be adsorbed is determined by the structure and distribution of pores in activated carbon, the structure and distribution of pores in activated carbon are important factors in adsorption.
Where is activated carbon used?

Activated carbon can be used in powdered or granular form, depending on the desired results. Granular activated carbon is commonly used in water treatment facilities to remove tastes, colors, odors, and dissolved organics by passing the water through a granular carbon bed.Because powdered carbon is a faster and better mechanical filter than granular activated carbon, it is the preferred choice in point-of-use water filtration systems.
For water treatment design and products, call Netsol Water.
Netsol Water is Greater Noida-based leading water & wastewater treatment plant manufacturer. We are industry's most demanding company based on client review and work quality. We are known as best commercial RO plant manufacturers, industrial RO plant manufacturer, sewage treatment plant manufacturer, Water Softener Plant Manufacturers and effluent treatment plant manufacturers. Apart from this 24x7 customer support is our USP. Call on +91-9650608473, or write us at enquiry@netsolwater.com for any support, inquiry or product-purchase related query.