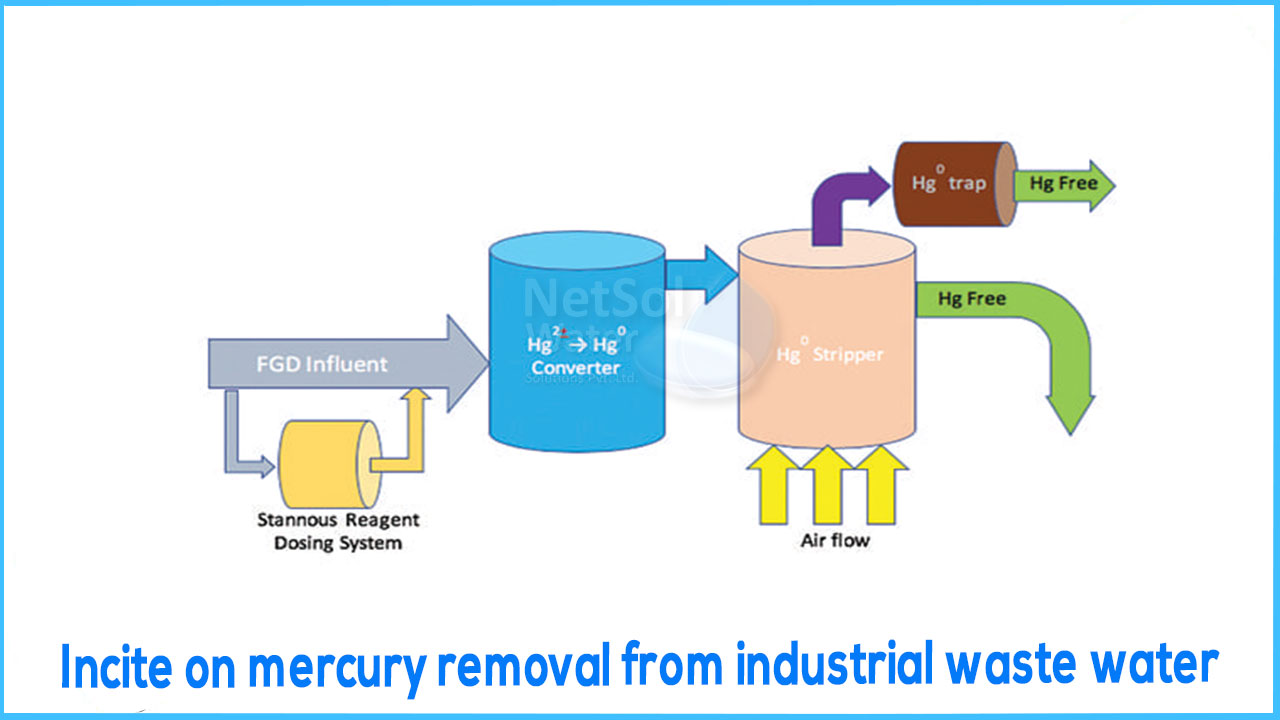
Mercury (Hg) is a well-known heavy metal that exists naturally as well as being introduced into the air and water through human activity. Mercury has been subject to tight limitations governing its use and disposal since the 1960s due to its high toxicity. Water treatment technologies are often used by industrial facilities that deal with mercury, such as those in the chloro-alkali and electronics sectors, among others, to minimize mercury levels in their liquid streams and emissions.
The physical and chemical makeup of wastewater, as well as its characteristics, can be complicated. The performance efficiencies of most treatment technologies are highly dependent on the water source (sediment, surface water, groundwater, or industrial effluent water) and water composition, including mercury speciation and concentration, water quality parameters such as pH, redox potential, ionic composition, ionic strength, and the presence of DOM and dispersed oil, and water quality parameters such as pH, redox potential, ionic composition, ionic strength, and the presence of DOM and dispersed oil. Several of these factors have been linked to the removal of mercury and other heavy metals.
Coagulation/Filtration, Granular Activated Carbon, Lime Softening, and Reverse Osmosis are four methods for removing mercury from normal water-
-COAGULATION/FILTRATION
It is a typical treatment that involves the use of AlSO4 to react with mercury and generate a solid that can be filtered out of the water. After that, the sludge must be dumped in a hazardous waste landfill. This method is advantageous because it is inexpensive and dependable.
-GRANULAR ACTIVATED CARBON
Granular activated carbon uses porous carbon media. This is a charcoal-based media with a lot of weight. The dissolved pollutants are absorbed and stored on the solid surface when the water passes through. Because the efficiency of this procedure is dependent on the amount of mercury in the water, it has some limits.
-LIME SOFTENING
Excess Ca(OH) is used in lime softening to raise the pH level, after which the heavy metal precipitate out as Hg (OH). Lower costs and proven reliability are two advantages of this technology.
-REVERSE OSMOSIS
In reverse osmosis, water is forced through a semipermeable membrane. Polyamide film is a common membrane material. This method generates high-quality water, but it is somewhat costly.
Precipitation, adsorption, membrane filtration, and biological treatment are the most prevalent methods for treating mercury-contaminated waste water. Each of these water treatment systems has its own set of benefits, which are highly dependent on the process parameters of a given installation.
1.CHEMICAL PRECIPITATION
Chemical precipitation is the most widely used water treatment method for removing mercury from both groundwater and wastewater since it is both cost-effective and simple to use. A facility will initially add a chemical precipitant to the stream to remove mercury by chemical precipitation. The precipitant reacts with dissolved elements in one of two ways to aid mercury removal: by generating insoluble elemental mercury or mercury compounds, or by forming particulate solids that adsorb dissolved mercury in the stream.After the precipitation reaction, the facility will use physical separation methods such as clarifying or filtering to remove insoluble solids from the liquid stream. Sulfides, ferric salts, ferric sulphates, and calcium hydroxide are the most common chemicals used to precipitate mercury, but other less common precipitants, such as lignin, are also utilized.
2.ADSORPTION
Adsorption is yet another water treatment process that is often used to remove mercury. Adsorption has several advantages, including no sludge production, high selectivity for mercury and/or other heavy metals, and a wide range of adsorption media materials. Adsorption, like precipitation, is capable of lowering mercury concentrations below 2 g/L.
Adsorption is a molecular attraction-based process in which a substance (adsorbate) accumulates on the surface of a solid (adsorbent). The technique usually involves passing a liquid stream across a bed of adsorbent material in water treatment applications.
3.MEMBRANE FILTRATION
Membrane filtration is a type of physical separation based on the size exclusion principle. The procedure involves passing a liquid stream across a semi-permeable membrane with precisely-sized pores that block larger particles and molecules while allowing smaller particles and molecules to pass through. As a result, membrane filtration is divided into four categories based on pore size: microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), and reverse osmosis (RO).
Membrane separation's key benefit is its high rate of mercury and other pollutant removal. This feature makes it a suitable choice for facilities that need to treat wastewater or process water for reuse, as well as those that must adhere to strict mercury or other heavy metals discharge limitations.
4.BIOLOGIC TREATMENT
Living microorganisms are used in biological treatment systems to break down and eliminate organic pollutants. While mercury is an inorganic substance, biological treatment can be employed to convert dangerous soluble mercury species into less soluble forms that are easier to remove or maintain. The cost-effectiveness of biological treatment is a major advantage, especially for streams with high mercury concentrations.Furthermore, biological treatment alone is less effective than other technologies at lowering mercury levels below strict limits. As a result, biological treatment is frequently followed by additional technologies like adsorption or precipitation.