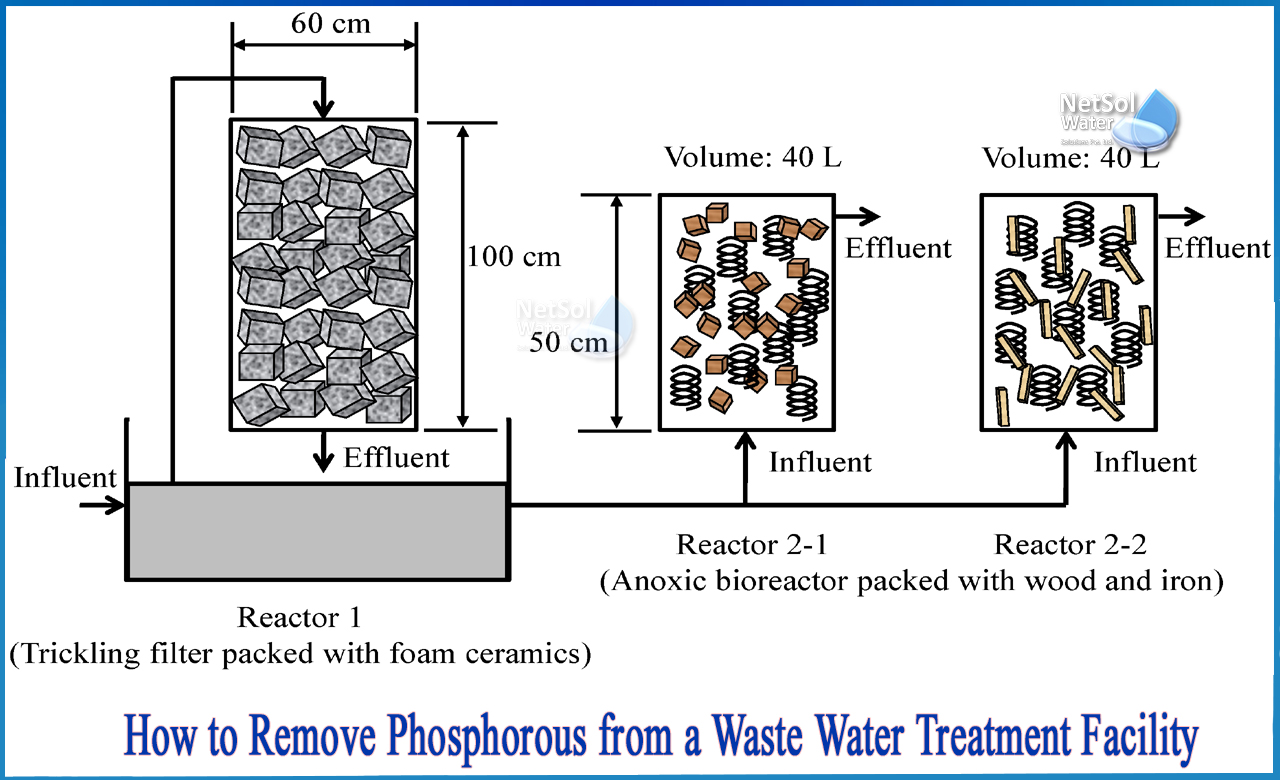
How to remove phosphorous from a Waste water treatment facility?
Chemical removal, advanced biological treatment, or a combination of both methods can be used to remove phosphorus from wastewater. The addition of calcium, iron, and aluminium salts to achieve phosphorus precipitation via various mechanisms is discussed in the chemical removal of phosphorus. Biological phosphorus removal, which is proposed as an alternative to chemical treatment, is based on the uptake of phosphorus in advance of actual bacterial metabolic requirements.
Phosphorous in waste water:
Municipal wastewaters may contain 5 to 20 mg/l of total phosphorous, 1-5 mg/l of which is organic and the rest is inorganic. Because phosphorous is a major component of synthetic detergents, individual contributions tend to rise. Individual phosphorous contributions range from 0.65 to 4.80 g/inhabitant per day, with an average of about 2.18 g.
Controlling phosphorous terminated from municipal and industrial wastewater treatment plants is critical to preventing surface water eutrophication. Phosphorus is a major nutrient that contributes to the eutrophication of lakes and natural waters. Its presence causes a variety of water quality issues, including increased purification costs, decreased recreational and conservation value of livestock loss, impoundments, and the potentially lethal effect of algal toxins on drinking water.
Phosphate expulsion is currently primarily accomplished through chemical precipitation, which is costly and causes a 40 percent increase in sludge volume. Biological phosphate removal is an ideal solution (BPR).
Removal processes of phosphorous:
Phosphorus removal from wastewater entails the incorporation of phosphate into TSS and subsequent removal from these solids. Phosphorus can be found in biological solids (such as microorganisms) or chemical precipitates.
1-Phosphate precipitation:
Chemical precipitation, which involves the addition of a coagulant and the mixing of wastewater and coagulant, is used to remove inorganic forms of phosphate. Calcium, aluminium, and iron are the most commonly used multivalent metal ions.
2-Calcium:
It is typically present in the form of lime Ca(OH)2. It reacts with the wastewater's natural alkalinity to produce calcium carbonate, which is primarily responsible for improving Suspend Solid removal.
Ca(HCO3)2 + Ca(OH)2↔2CaCO3 ↓+ 2H2O
When the pH of the wastewater exceeds about 10, excess calcium ions react with the phosphate and precipitate in hydroxyl apatite.
10 Ca2+ + 6 PO43- + 2 OH- ↔ Ca10(PO4)*6(OH)2 ↓
Because the reaction is between the lime and the alkalinity of the wastewater, the amount needed will be independent of the amount of phosphate present. It will be primarily determined by the alkalinity of the wastewater. The lime dose required is roughly 1.5 times the alkalinity as CaCO3. To reduce pH before further treatment or disposal, neutralisation may be required. Carbon dioxide (CO2) re-carbonation is used to lower the pH value.
3-Aluminium and iron:
Alum, also known as hydrated aluminium sulphate, is widely used in the precipitation of phosphates and aluminium phosphates (AlPO4). The basic reaction is as follows:
Al3 + HnPO43-n ↔ AlPO4 + nH+
This reaction appears to be simple at first glance, but it must be considered in light of the numerous competing reactions and their associated equilibrium constants, as well as the effects of alkalinity, pH, and trace elements found in wastewater. The dosage rate required is determined by the amount of phosphorous that must be removed. Coagulation efficiency decreases as phosphorous concentration decreases.
In practise, coagulant dosage rates ranging from 50 to 200 mg/l result in an 80-90 percent removal rate. Dosages are generally established using bench-scale tests and, on occasion, full-scale tests, especially when polymers are used. At dosage rates greater than 150 mg/l, aluminium coagulants can have a negative impact on the microbial population in activated sludge, particularly protozoa and rotifers.
4-Ferric chloride:
Although the actual reactions are not fully understood, ferric chloride or sulphate, and ferrous sulphate, also known as copperas, are all widely used for phosphorous removal. The basic reaction is as follows:
Fe3+ + HnPO43-n ↔ FePO4 + nH+
Ferric phosphate is formed when ferric ions combine. They react slowly with natural alkalinity, so a coagulant aid, such as lime, is usually added to raise the pH and improve coagulation.
For more information, contact Netsol Water.
Netsol Water is Greater Noida-based leading water & wastewater treatment plant manufacturer. We are industry's most demanding company based on client review and work quality. We are known as best commercial RO plant manufacturers, industrial RO plant manufacturer, sewage treatment plant manufacturer, Water Softener Plant Manufacturers and effluent treatment plant manufacturers. Apart from this 24x7 customer support is our USP. Call on +91-9650608473, or write us at enquiry@netsolwater.com for any support, inquiry or product-purchase related query.