WHAT DO A WATER SOFTENER REMOVE?
Water softeners purify hard water by removing calcium and magnesium ions. The two minerals that cause water hardness are calcium (Ca2+) and magnesium (Mg2+). In addition, any positively charged ion will be attracted and eliminated during the ion exchange process (also known as a cation). Other minerals, such as iron and manganese, may be included.
Water softeners remove ferrous iron (dissolved iron) when it is present in small amounts and the majority of the iron is soluble. Iron darkens the colour of water and causes visible stains on your toilet, bathtub, and sinks. The removal of ferric iron (insoluble iron) with a softener is more difficult. Ferric iron will accumulate on the resin bed and resist the regeneration cycle's backwashing. This can result in iron slugs in your softened water, reducing the potency of the resin beads. When dissolved iron comes into contact with oxygen, it oxidises and transforms into ferric iron.
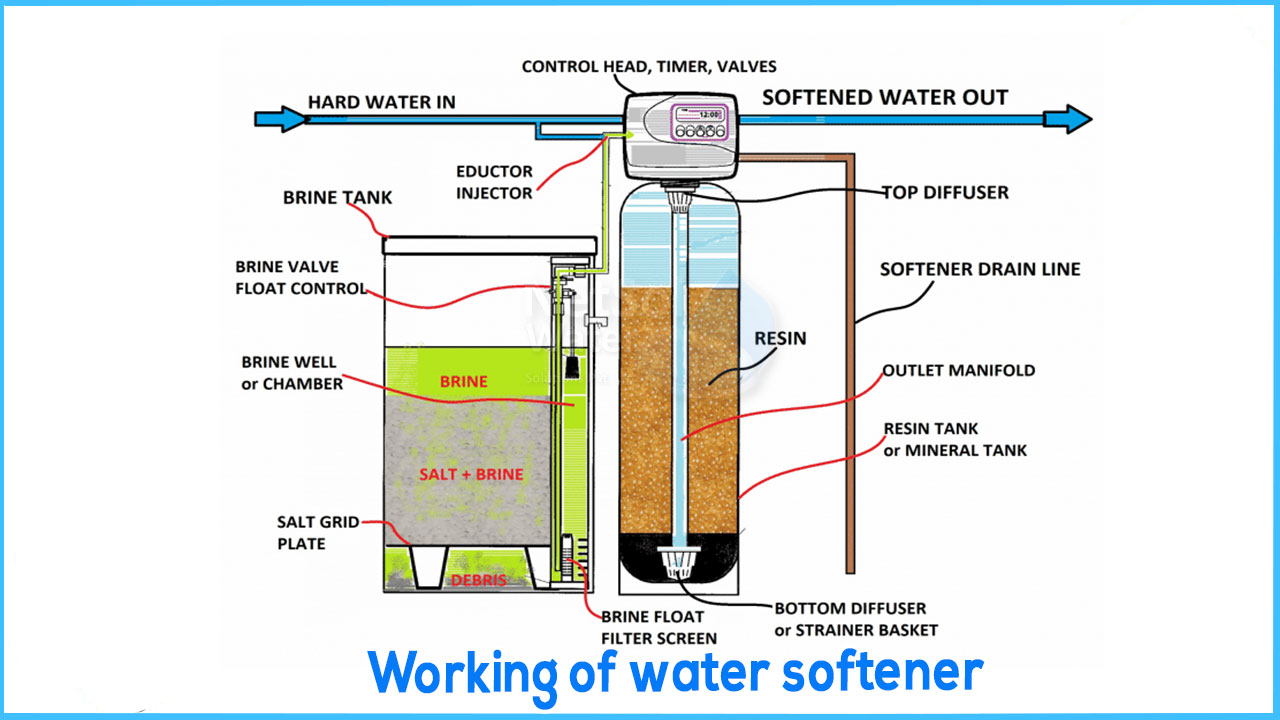
HOW DO WATER SOFTENER WORKS?
Ion exchange is the method by which water softeners remove calcium and magnesium from the water. Hard water travels through a bed of spherical resin beds as it enters the mineral tank. These sodium-ion-charged plastic beds are typically constructed of polystyrene. The resin beds carry a negative charge, making them anions. Calcium and magnesium are cations because they have a positive charge. The negative charge of the minerals is attracted to the positive charge of the resin beds because opposing charges attract. The beds take hold of the mineral ions and remove them from the water as the hard water travels through the resin.
HOW DOES WATER SOFTENER REGENERATION WORKS?
During regeneration cycles, a highly concentrated brine solution is poured over the resin beds, washing away the hardness minerals and draining them out of the system. To remove the hardness minerals, the resin beds are recharged and primed. Resin beds are incredibly long-lasting and can soften your water for up to twenty years. One of two procedures is used to renew water softeners: Regeneration that is either co-current or counter-current.
CO-CURRENT REGENERATION WORK: The brine solution enters the mineral tank in the same direction as the service flow in a co-current regeneration cycle. The brine solution runs down the depth of the resin bead bed, causing an ion exchange process to occur in reverse. The salts drive the beads to release magnesium and calcium ions in exchange for sodium ions as the brine flows over them. As the brine passes through the resin, a rush of hardness minerals forms and circulates throughout the system, becoming increasingly concentrated. Minerals and regeneration ions are continuously exchanged and re-exchanged as the brine solution forces more hardness minerals through the bed. The strength of the solution has been greatly diminished by the time the water has exited the tank. The most charged beds will be on the ones near the top of the tank in a co-current regeneration cycle. To complete the regeneration process, co-current regeneration takes more water and salt than counter-current regeneration.
COUNTER-CURRENT REGENERATION CYCLE: Water enters the tank through the bottom of the mineral tank, where it generally escapes, in a counter-current regeneration cycle. The brine is pumped up the resin bed in a countercurrent cycle, starting at the bottom, where the resin beds are normally the least depleted. This indicates that throughout the regeneration cycle, fewer hardness minerals initiate re-exchange. When the brine reaches the top of the resin bed, where the softener first comes into touch with the hard water, it is less depleted. In comparison to co-current cycling, a counter-current cycling water softener consumes 75% less salt and 65% less water. It also more evenly distributes the recharging sodium ions. The most highly charged beds will be near the bottom of the tank, shortly before the water exits into the house, in a countercurrent cycle. High-efficiency water softeners are another name for these.