Introduction
Almost every sector requires water and wastewater treatment at some point throughout the manufacturing process. In order to determine the efficiency of each process, maintain overall quality, and ensure that operations fulfill the tight regulatory standards governing them, measuring the quality of both influent and effluent water is critical.
Measuring and evaluating pH levels is one of the most important indicators for ensuring that these standards are met. Although pH measurement is most generally linked with the water treatment industry, it is used in a wide variety of sectors. In the food business, for example, it can affect the taste and shelf life of a product. pH measuring can also be used in other industries, such as textiles, pulp and paper industry, agriculture, etc.
What is pH?
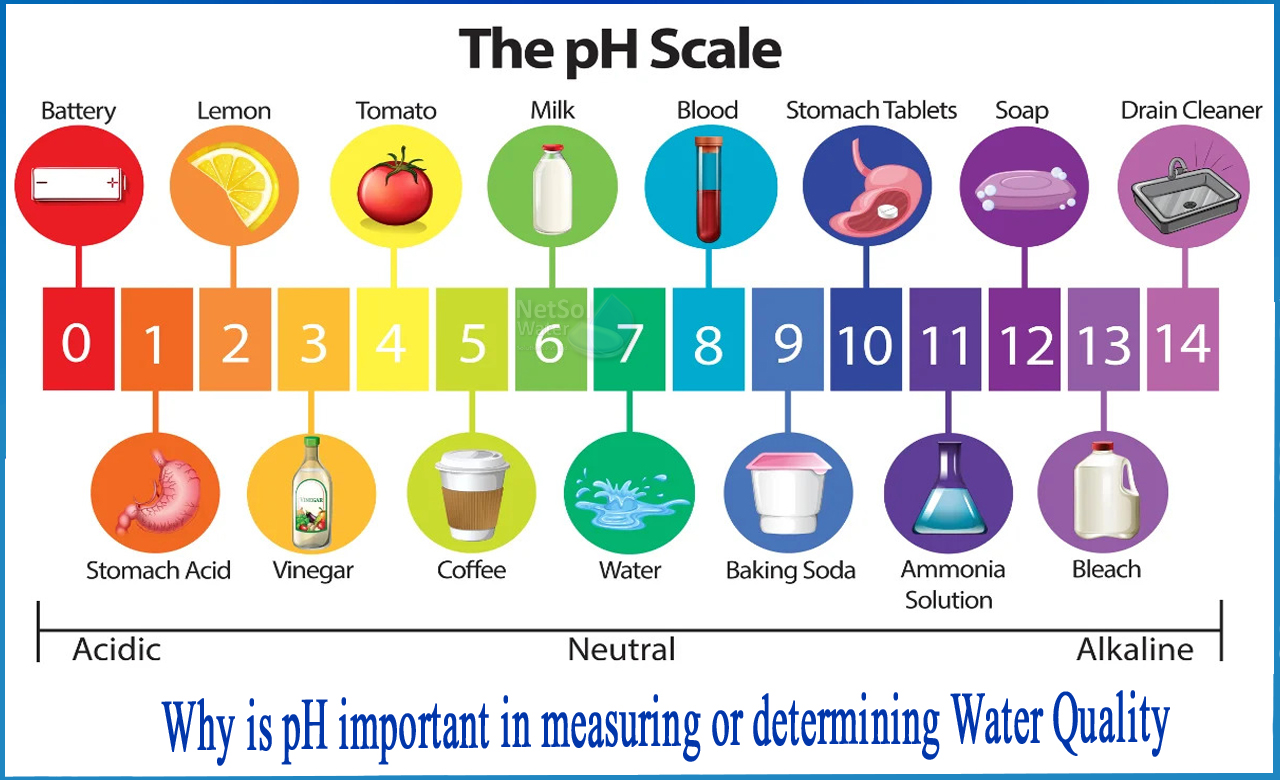
The pH of water is a measurement of its acidity or basicity. pH is measured on a scale of 0 (pure acid) to 14 (pure alkaline solution). The pH of distilled water is 7 and is neutral. A tenfold difference in acidity is represented by each unit on the pH scale.
When certain compounds are dissolved in water, they form charged molecules known as ions. Acidic water has more hydrogen ions (H+) and has a pH of 0-7, whereas basic or alkaline water contains more hydroxyl ions (OH-) and has a pH of 7-14.
Importance of pH
The pH of water determines the solubility (the amount that can be dissolved in water) and biological availability (the amount that can be used by aquatic life) of chemical components such as nutrients and heavy metals. pH, for example, affects not just how much and what type of phosphorus is present in the water, but also whether aquatic life can utilize it. The degree to which heavy metals are soluble determines their toxicity. Metals are more harmful at lower pH because they are more soluble.
Why is pH important in measuring or determining water quality?
With a pH of 7, pure water is regarded a neutral. Regrettably, not all water is clean, and it is rarely at this level. The acidity and alkalinity of water can be affected by a variety of causes. The first and most obvious of them is the water's location in the bedrock and soil composition.
Acidity in the water may be mitigated depending on the rock type. Plant growth and organic material near water, on the other hand, can actually increase acidity due to carbon dioxide emissions during decomposition. Chemical dumping, acid precipitation, and coal mine drainage are among the other causes.
pH levels that are too high or too low might have a negative impact on water use.The pH of drinking water is regularly controlled, and the Environmental Protection Agency requires it to be kept within a range of 6.5–8.5. Drinking acidic or alkaline water, however, can be dangerous if not adequately controlled.
Conclusion
Water with a pH of less than 7 has a corrosive character to it. This means iron, copper, lead, or zinc from plumbing and other metal fittings could be present. It will be harsh and metallic in flavor. Although high alkaline water poses no health dangers, it does produce aesthetic issues. Scale or precipitate will form on the pipework, fittings, plates, and utensils. The taste will be similar to baking soda, and the texture will be slick.
Now, are you looking for the most effective solution for your water or wastewater?
If that's the case, contact Netsol Water! Our WWT solution purifies wastewater so that it may be reused and safe water can be released into the environment. For effluent treatment, we have a dedicated team of experts. Because each industry produces different types of waste water, the process is tailored to meet the needs of each client.
If you need any technical assistance and guidance, or simply have a query regarding our water and wastewater treatment technology solutions,
You may reach us via phone at +919650608473 or by email at enquiry@netsolwater.com