Overview
Many water and wastewater treatment solutions rely on oxidation as a driving force. One solution in particular is focused on maximizing the system's oxidation potential. The advanced oxidation process is the name given to this particular procedure.
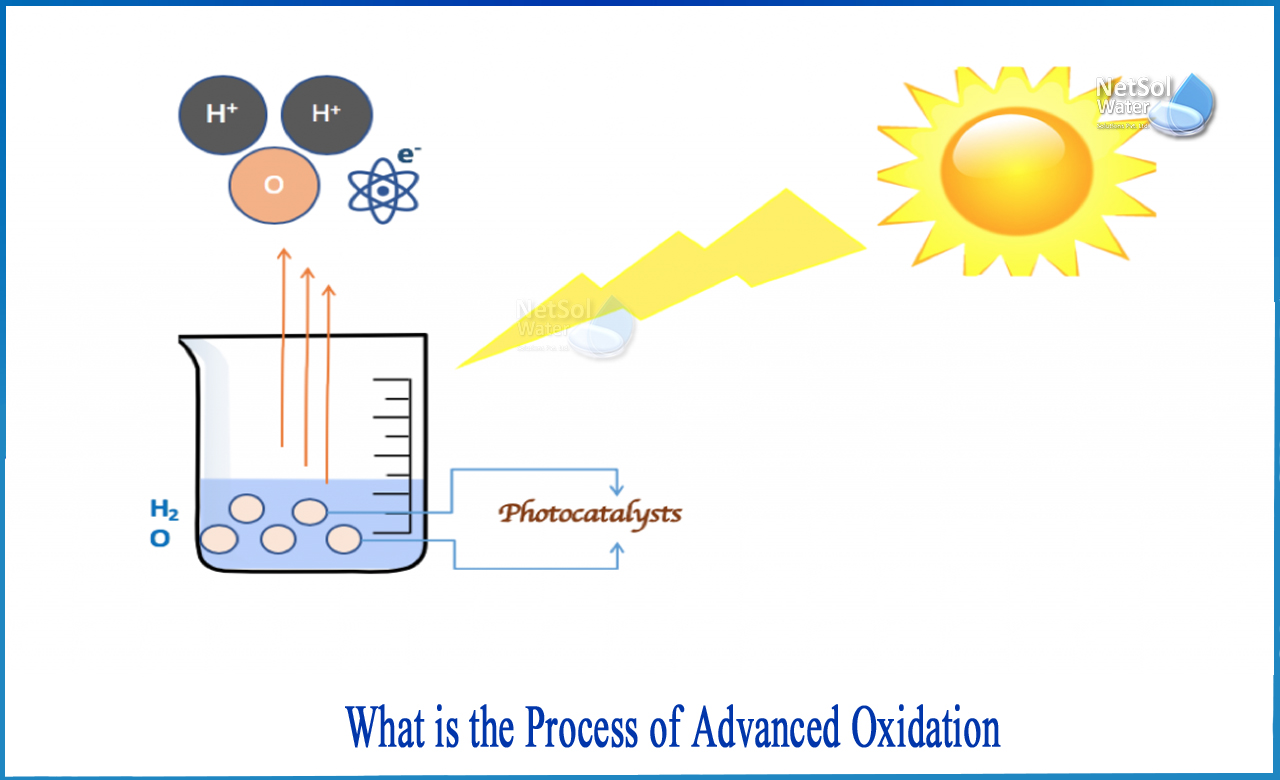
What is the process of advanced oxidation?
The term "advanced oxidation" typically refers to processes that generate hydroxyl radicals (OH). The primary oxidants in this process are radicals, which break down compounds into intermediates and then mineralize those intermediates into simple compounds like water, carbon dioxide, and salts.
In general, these molecules are formed as specific compounds degrade, specifically ozone (O3) and hydrogen peroxide (H2O2). Other components, such as ultraviolet light (UV), are used as catalysts in the reaction to encourage the compounds to degrade appropriately.
However, as with many water/wastewater treatment solutions, each option has advantages and disadvantages. As a result, it is critical to carefully select a process that will work best with your specific application.
How does one make such a decision?
By understanding some of the fundamentals of this process
Types of advanced oxidation processes:
1: Ozone
In an alkaline solution, ozone interacts with hydrogen-containing compounds and decomposes in a series of steps to reduce to OH radicals. It can be used as an AOP on its own, but only at higher pH levels due to the abundance of hydroxide ions present. Though the reactions are much slower, O3 is also a powerful oxidant and acts as a secondary oxidizer in the overall process. Because O3 has a very short half-life, it must be generated on-site and used quickly if it is used. Furthermore, if bromide is one of the contaminants in the wastewater, the formation of bromates molecules, which are highly toxic, is possible.
2: Hydrogen Peroxide
Hydrogen peroxide, unlike ozone, cannot be used as a stand-alone oxidation treatment. It is not as effective as O3 as a secondary oxidizer, but it can react with hydrogen and oxygen-containing compounds in a less complicated manner than ozone. It does not need to be manufactured on-site, but it must be carefully stored because it is unstable. H2O2 must also be monitored for residuals that remain after treatment. Because the compound can be toxic to humans, it may need to be treated.
3: Ultraviolet Light
Because of its ability to kill or prevent the reproduction of a variety of pathogens, ultraviolet light is widely used as a disinfectant. UV is not an oxidant in and of itself because it is just a wavelength of light, but it does transfer massless photons to chemical compounds, breaking their bonds quickly and easily. However, because UV interaction is light-driven, some contaminants, such as suspended solids, can reduce its efficiency by blocking it from the target compounds.
4: Combinations
The following treatments are frequently used in conjunction with one another:
O3/UV, O3/H2O2, H2O2/UV, and O3/H2O2/UV.
These combinations leverage the strengths of the individual processes to improve the overall efficiency of the advanced oxidation process. However, each combination has some disadvantages; thus, the optimized process is chosen based on application.
Following that, it is important to discuss what factors must be considered when selecting the best process for a specific application.
1: Composition of water
The composition of the influent water, perhaps most obviously, should be carefully considered. Because this process is a complex chemical process, the decision on which process to use is influenced by the specific pollutants present in the water to be treated.
2: Treatment objectives
The amount of water/wastewater that must be treated is determined by environmental regulations or reuse considerations. More lax standards may only necessitate a simple process, whereas stricter standards may necessitate something a little more robust.
3: UV exposure
As with any other UV disinfection process, an appropriate amount of UV exposure is required to achieve the desired results without consuming excessive amounts of energy, rendering this process uneconomical.
4: Dose of chemical
Adequate doses of O3 and/or H2O2 must be added in order to achieve an acceptable concentration of OH radicals. Again, this is a chemistry-intensive process, and chemistry necessitates proper dosing or else the desired results will not be obtained.
5: Consumption of energy
In some cases, the process can be an energy-intensive system, with certain system configurations or applications requiring more than others.
6: Cost
These process systems are generally more expensive, but some systems are more expensive than others. The most significant costs are associated with operational aspects such as keeping up with the demand for input chemicals and energy based on contaminant levels.
Netsol Water is Greater Noida-based leading water & wastewater treatment plant manufacturer. We are industry's most demanding company based on client review and work quality. We are known as best commercial RO plant manufacturers, industrial RO plant manufacturer, sewage treatment plant manufacturer, Water Softener Plant Manufacturers and effluent treatment plant manufacturers. Apart from this 24x7 customer support is our USP. Call on +91-9650608473, or write us at enquiry@netsolwater.com for any support, inquiry or product-purchase related query.