Treatment of wastewater and industrial process water typically involves the electrolytic process known as electrocoagulation (EC). It is one of the fastest-growing technologies in this field because of its exceptional capacity, to remove pollutants such as emulsified oils, suspended particles, and dissolved metals.
Let’s explore the concept of electrocoagulation in STPs and its application areas in this blog.
What is Electrocoagulation technology in STPs?
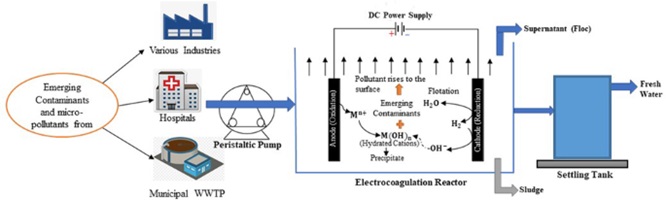
The reactor that electrochemically processes the wastewater is called an electrocoagulation reactor. The treatment will get rid of the COD particles, BOD particles, silica, TSS, turbidity, heavy metals, hardness, nitrogen components, phosphate, and colour. The coagulation flocs will collect all of these impurities, which will then be removed from the water by flotation or sedimentation.
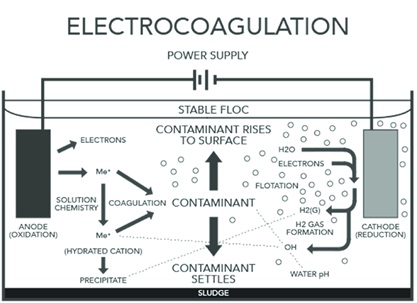
Components of Electrocoagulation systems
The electrocoagulation unit typically consists of:
· Filtration
· Neutralisation
· EC reactor
· Coagulation vessel
· Unit for flotation or sedimentation
· A container for storing salt or chemicals and dosing pumps
· Systems for dewatering and external filter press
Working of EC Reactor in STPs
In contrast to some other electrolytic processes, the anode in electrocoagulation is made of a corrosive metal, typically iron or aluminium. The anode begins to oxidise and corrode into solution as metal cations, once it is linked to the power source. At the same time, hydroxyl groups and hydrogen gas are formed from water at the cathode. Large flocs are created when metal ions and hydroxyl groups combine, entrapping impurities such as emulsified oils, suspended particles, and other pollutants. Additionally, the released ions help to enhance coagulation by balancing the surface charges of particles.
Firstly, the dissolution of sacrificial Fe (0) anodes causes the in situ formation of Fe hydroxide salts (Fe precipitation), when electric current is applied to Fe plates (the same occurs for Al). Fe (0) oxidises to dissolved Fe (II), which then undergoes further oxidation to become Fe. (III).
Secondly, at the cathode, water is converted into hydrogen gas (H2) and hydroxyl ions (OH-), which can mix with metal ions to produce metal hydroxides. Since, electron transfer restricts the formation of these species, the rate of species formation is inversely proportional to the applied current intensity. Metal ions and metal hydroxides are then used as coagulants in the coagulation process after they have formed.
Thirdly, because they are heavier than water, the newly produced flocs settle to the bottom of the tank. To meet the necessary water quality goals, flocculated particulates are separated from the supernatant water, using subsequent sedimentation and filtration operations.
Lastly, the anodes will totally disintegrate during the coagulation process and over time, necessitating replacement. In certain systems, the entire anode-cathode series will be taken out of the tank and replaced with a fresh set.
Applications of electrocoagulation in STPs
The EC technology can be applied to the following type of industries:
- Textile
- Pulp and paper
- Automotive
- Chemical and pharmaceutical
- Cosmetics
- Detergents
- Airports
- Painting
- Solid waste
- Plastic
- Breweries and wineries
- Slaughterhouses
- Domestic
- Hotel
- Fruits and vegetables
- Ballast water treatment
- Cooling towers
High quality sewage treatment systems from Netsol
Clean water is produced by Netsol Water EC treatment systems, which are designed, built, and installed with safety and the environment in mind. Our EC units can be constructed according to the needs of the customer.
Since, our quality management system has been approved, therefore, we welcome client inquiries regarding the premium, pure water they require for their particular purposes. Our technical experts can accurately plan and create the device, exceeding client quality requirements. For further information, contact us by phone at +91 9650608473 or by email at enquiry@netsolwater.com.



