REDOX REACTION
Redox reactions are oxidation-reduction chemical reactions in which the reactants undergo a change in their oxidation states. The term ‘redox’ is a short form of reduction-oxidation. All the redox reactions can be broken down into two different processes – a reduction process and an oxidation process.
The oxidation and reduction reactions always occur simultaneously in the redox reaction or Oxidation-Reduction reaction. The substance getting reduced in a chemical reaction is known as the oxidizing agent, while a substance that is getting oxidized is known as the reducing agent.
PURPOSE
The purpose of redox reaction is used in the treatment of all types of water for a range of purposes:
- 1. Disinfection before household or industrial using in order to avoid any danger of bacterial contamination;
- 2. Precipitating dissolved compounds (iron, manganese, sulphides);
- 3. Breaking down organic compounds and especially those responsible for colour, odour and taste in water, those that are toxic and, more generally, those that contribute to the water’s chemical oxygen demand;
- 4. Eliminate ammonia nitrogen;
- 5. Converting non-biodegradable pollution into substances that can be assimilated by bacteria in a subsequent biological treatment.
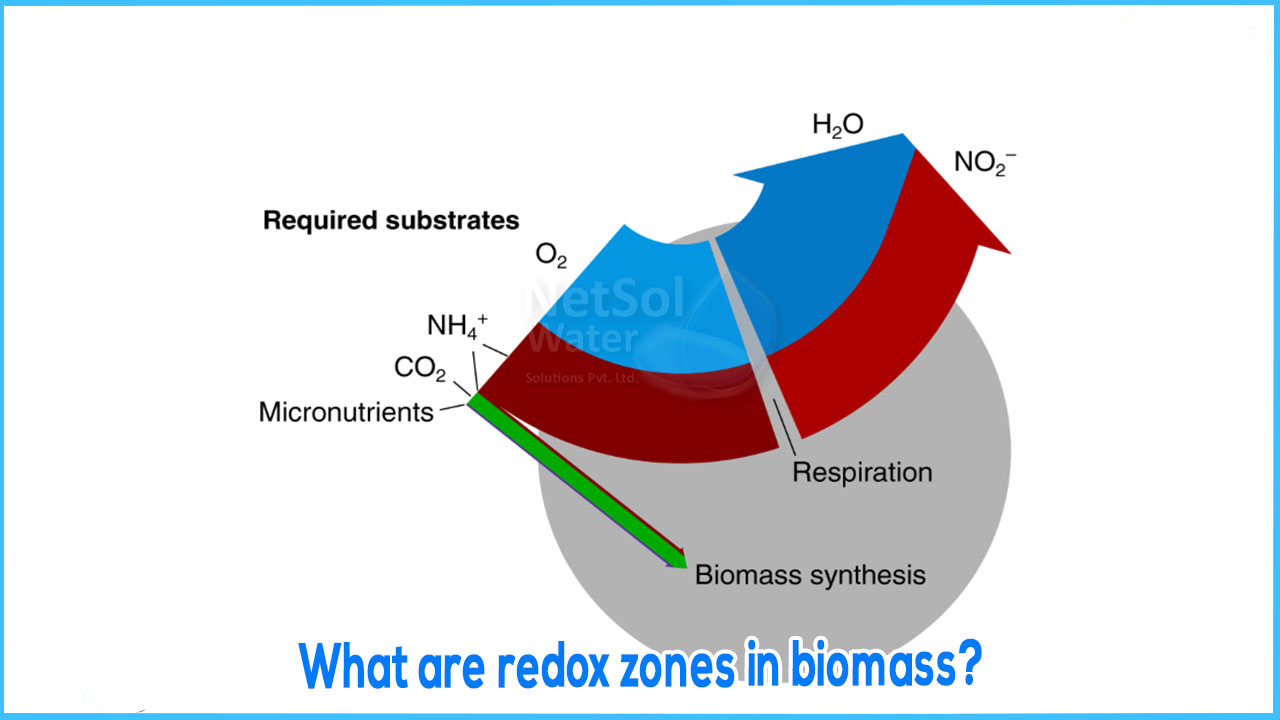
Since biomass is present in the form of flocs or biofilms, the removal rates are reduced due to limited diffusion. This is primarily dominant in bio filters and to some extent in activated sludge plants as well. It is vital to understand the phenomenon that the diffusion hinders the reaction rate inside of the filter; it is equally important to understand that it may lead to redox zones.
REDOX ZONES
The illustration of different redox zones in a bio film has been shown for clear understanding. While considering these key points one must get a clear picture from the picture shown. Each layer indicates whether the substance has been removed or produced. In zones where in the curvature is negative the substance stands removed where as in zones with curvature as positive, the substance stands produced.
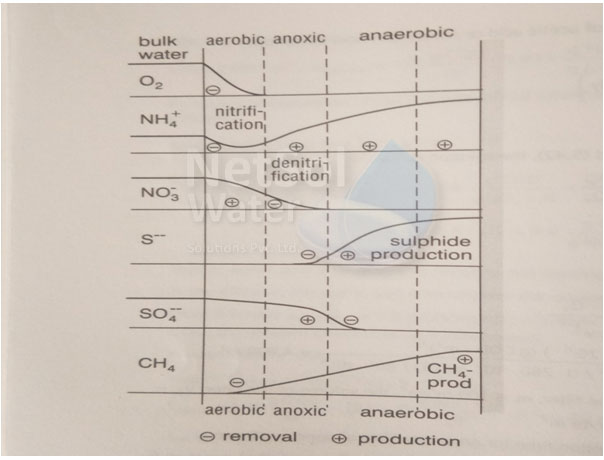
The outer layer is oxygen rich water and thereby is aerobic in nature. It is the penetration depth of the oxygen which limits the removal (predominantly aerobic). In this zone the substances can be degraded aerobically and are oxidized.
If nitrate is present in the water and if nitrate is produced by nitrification in the aerobic zones, nitrate gets diffused in oxygen zone where nitrification will occur, this zone is an anoxic zone. In the internal, reduced zones, the all relevant processes take place under aerobic conditions. They may comprise of well-known redox processes.In context to the anaerobic degradation, a release of ammonium takes place which gets diffused into aerobic zones. The ultimate end is reduction of organic matter to methane and carbon dioxide in this zone.
All these processes are acidity and alkalinity producing and will simply influence the hydrogen ion concentration in the floc or biofilm which is of utmost importance in redox reactions. The other factors which affect the reaction are hydrodynamics viz. sloughing and new growth etc.Comparatively very little is known about these conditions and their importance to design and optimize the biological water treatment process.