What is Electrodeionization or EDI Plant?
Electrodeionization (EDI) is a continuous, chemical-free process of removing ionized and ionizable species from feedwater using DC power. Reverse osmosis (RO) permeate is often polished using EDI, which is an innovative substitute for and efficient replacement for traditional mixed bed ion exchange (IX).
EDI can also be referred to as continuous electrodeionization (CEDI) as the electric current regenerates the ion exchange resin mass continuously. Three distinct techniques are combined to create electrodeionization (EDI):
Electrolysis: The process of electrolysis pulls anions and cations from diluting chambers through cation or anion exchange membranes and into concentrating chambers by using a continuously applied electric DC current to direct both positive and negative ions to the electrode that has the opposite electrical charge.
Ion exchange: Ion exchange is a process in which the diluting chambers are made of ion exchange resin, and chemical regeneration, in which water splitting occurs as the cations and anions are attached to resin sites as water passes through the resin bed.
Chemically regenerated mixed bed: in a chemically regenerated mixed bed, the hydrogen (H+) of acid regenerates cation resin. Anion resin is renewed using sodium hydroxide (caustic soda) hydroxide (OH-). In EDI, the electrical current splits water into H+ and OH-, renewing the resin without the use of external chemicals.
Design basis:
Ion-Exchange Membranes: These components are at the centre of the EDI process. Anionic and cationic exchange resins are present in the polymeric substance that makes up these membranes. The resins in the membranes pull the ions out of the water when an electrical current is passed through them.
Electrical Current: The electrical current is utilised to establish a concentration gradient across the ion-exchange membranes. The ions can be drawn out of the water and collected on both sides of the membrane thanks to the concentration gradient.
Polishing Cartridge: To eliminate any last traces of pollutants from the filtered water, use the polishing cartridge. Activated carbon, mixed bed resin, or other media that may remove any leftover particles are frequently included in the polishing cartridge.
How does EDI work?
The basic design of an EDI stack is similar to that of a deionization chamber. Ion exchange resin is housed in the chamber sandwiched between cationic and anionic exchange membranes. The membrane blocks water flow, allowing only ions to pass through.
Several processes start when flow enters the resin-filled diluting chamber. The resins in the mixed bed scavenge strong ions from the feed stream. Charged ions are driven away from the resin and towards the corresponding, oppositely charged electrodes by the intense direct current field that is applied across the stack of components. This method allows for the continual removal and transfer of these charged strong ion species into the adiacent concentrating compartments.
The concentration chamber allows the ions to pass through as they move in the direction of the membrane, but it prevents them from reaching the electrode. The neighbouring membrane, which has a resin with the same charge, blocks them.
The conductivity of the process stream drops significantly as the strong ions are eliminated. The resin beads' surface water is split by the intense electrical potential being applied, releasing hydrogen and hydroxyl ions. These function as ongoing regenerators for the ion-exchange resin. The ionisation of neutral or weakly ionised aqueous species, like carbon dioxide or silica, is possible with the help of these regenerate resins. Ion exchange membranes and direct current are used to remove ions after ionisation.
The following is a list of the ionisation reactions that take place in the resin in hydrogen or hydroxide forms to remove weakly ionised compounds:
CO2 + OH- ==> HCO3-
HCO3- + OH- ==> CO32-
SiO2 + OH- ==> HSiO3-
H3BO3 + OH- ==> B(OH)4-
NH3 + H+ ==> NH4+
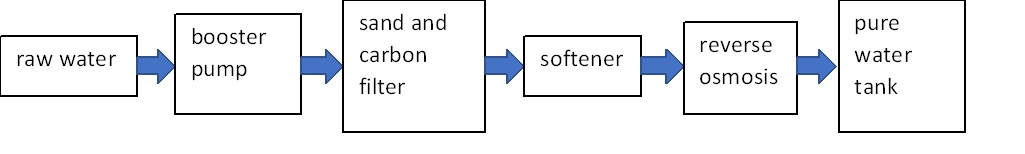
Applications of EDI water treatment plant:
1. Reusing used water in the food and beverage industries Manufacturing chemicals
2. Biotechnology
3. Electronics
4. Cosmetic Laboratories
5. Industry of pharmaceuticals
6.Water Used for Boilers
7. Ionizable SiO2 and TOC (total organic carbon) reduction
Do you need an advice or assistance on selecting the best water and waste water treatment unit? We have solutions for all your problems!
Let us now your problem, our experts will make sure that it goes away.
For an assistance or related query,
Call on +91-965-060-8473
Or write us at enquiry@netsolwater.com



