What is Total Kjeldahl Nitrogen in Waste Water Treatment?
The Total Kjeldahl Nitrogen (TKN) teststwo measurements together, i.e. measurement of organic nitrogen and ammonia nitrogen concentrations into a single value. As a result, in order to determine the organic nitrogen concentration, a separate ammonia test must be conducted, the results of which are deducted from the TKN value.
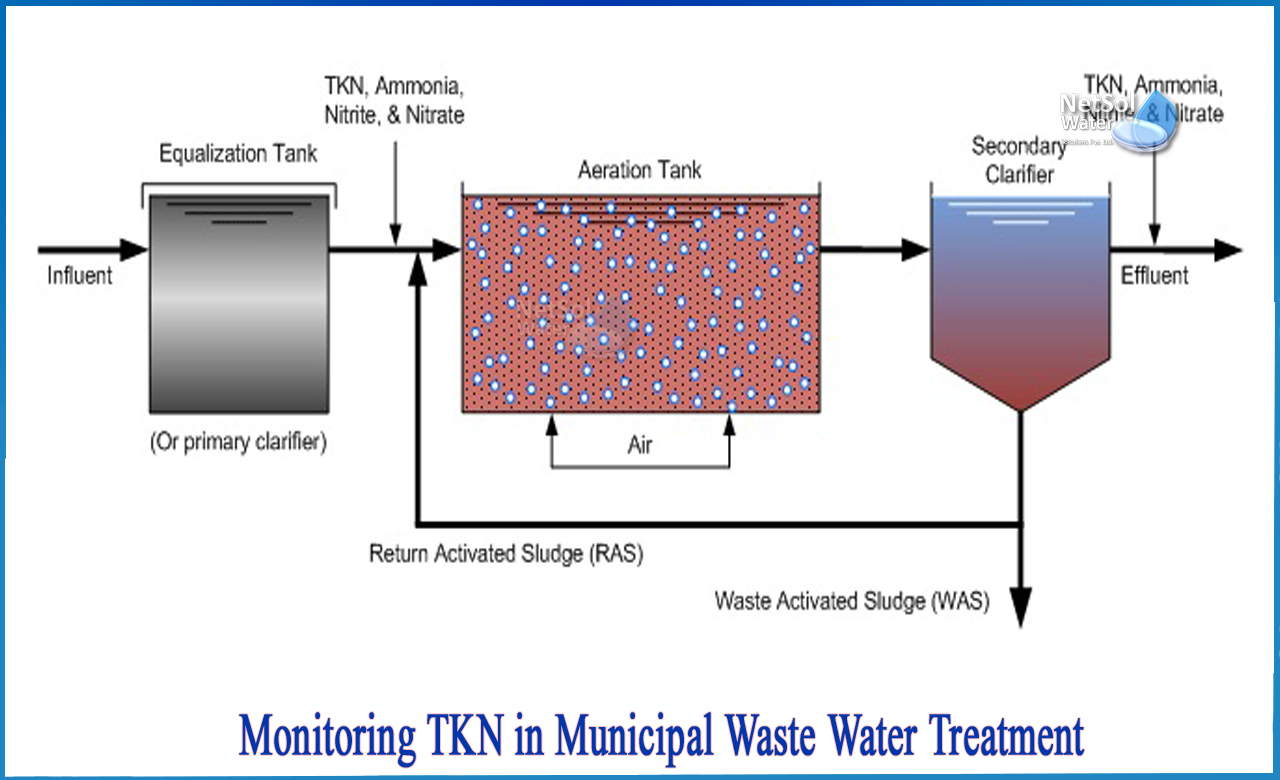
When the effluent ammonia concentration rises, it may appear that ammonia oxidation (nitrification) has decreased in refinery biological treatment systems with highly variable ammonia loadings. However, this may not be the case. Because amines and other organic nitrogen compounds are converted to ammonia after the influent sample point, the effective oxidation of ammonia is, in a sense, hidden.
When the influent TKN concentration exceeds the ammonia concentration (TKN will always be ammonia nitrogen), determining nitrification efficiency is impossible because ammonia is added to the wastewater from the breakdown of organic nitrogen at the same time ammonia is eliminated (nitrified) in the bioreactor. Knowing the TKN and ammonia concentrations in the influent and effluent will give a better idea of how the biological system is handling (nitrifying) the applied ammonia load.
Nitrogen is initially present in freshly polluted water as organic nitrogen and ammonia. Organic nitrogen is gradually converted into ammonia by natural biochemical processes, which is the best form of nitrogen for microorganisms in the treatment process to use as a nutrient. When nitrite and ammonia nitrogen concentrations are at or near zero and nitrate concentrations are at or near maximum, the wastewater has been fully nitrified. Organic nitrogen will be present in very small amounts in fully nitrified wastewater.
Organic nitrogen measurement is difficult because the sample must be digested prior to analysis in order to convert the organic nitrogen into a form that is more amenable to analysis. A Kjeldahl digestion needs to convert organic nitrogen to ammonia, but it necessitates the collection of a distillate, which is difficult for a field-based on-line analyser to do.The persulfate digestion method converts all nitrogenous compounds to nitrate, but it necessitates heating the sample for 30 minutes at 110O C with a digestion reagent before cooling to room temperature for analysis. In the field, this is also difficult to automate.
Testing method for TKN:
Total Kjeldahl Nitrogen (TKN) analysis determines both organic and inorganic nitrogen forms. The analysis begins with an acid digestion of the organic samples, which converts organic nitrogen to ammonia. To convert organic nitrogen to ammonia, the sample must be boiled in concentrated sulphuric acid, potassium sulphate, and a copper catalyst. By raising the digestion temperature to 395° C, the rate of this reaction is increased. As a result, this digestion procedure must take place within a fume hood. The second part of the method is a repeat of the distillation described above, but the pH of the acidic digestion sample must be raised to 9.5 with concentrated sodium hydroxide. Ammonia gas is formed at this pH, and the gas is transferred by distillation to the acidic trapping/absorbing solution, where it is converted back to ammonium.
The concentrations of nitrogen in the receiving solution can then be determined using titrimetric, colorimetric, or specific ion electrode techniques.
The above method, as well as its various forms of quantitative nitrogen analysis within the collected distillate, is well recorded in EPA, AOAC, AACC, ASTM, and Standard Methods for Examination Water and Wastewater compendiums.
After determining the total nitrogen, the inorganic nitrogen fraction value can be subtracted to resolve only the organic nitrogen fraction.
TKN = Organic N + Ammonia N
Organic N = TKN - Ammonia N
Nitrogen sources such as azide, azo, hydro zones, nitrile, semicarbozones, and oximes are not recovered in the TKN procedure described above.
It is important to note that Kjeldahl nitrogen digestion and distillation determination equipment is available in three sizes. Each allows the operator to perform the same nitrogen analysis digestion and distillation chemical procedures. The digestion and distillation sample vessels, on the other hand, shrink as the size of the Kjeldahl equipment shrinks. (Macro = 800 ml, Block = 250 ml, and Micro = 100 ml sample vessels). Accurately and repeatedly detecting low nitrogen concentration levels is frequently dependent on the initial sample volume. Larger sample sizes, in general, improve the precision and accuracy of low level nitrogen analysis.
For more information, contact Netsol Water.
Netsol Water is Greater Noida-based leading water & wastewater treatment plant manufacturer. We are industry's most demanding company based on client review and work quality. We are known as best commercial RO plant manufacturers, industrial RO plant manufacturer, sewage treatment plant manufacturer, Water Softener Plant Manufacturers and effluent treatment plant manufacturers. Apart from this 24x7 customer support is our USP. Call on +91-9650608473, or write us at enquiry@netsolwater.com for any support, inquiry or product-purchase related query.