What is an advanced oxidation process?
Advanced oxidation process is a water treatment technique that treats water at the third stage of an integrated water treatment system by using the chemical reaction of oxidation.
The goal is to drastically limit the amount of micropollutants that make their way past the primary and secondary stages. Certain AOP systems may be able to treat pollutants more successfully depending on the water quality. Choosing one can be a lengthy procedure.
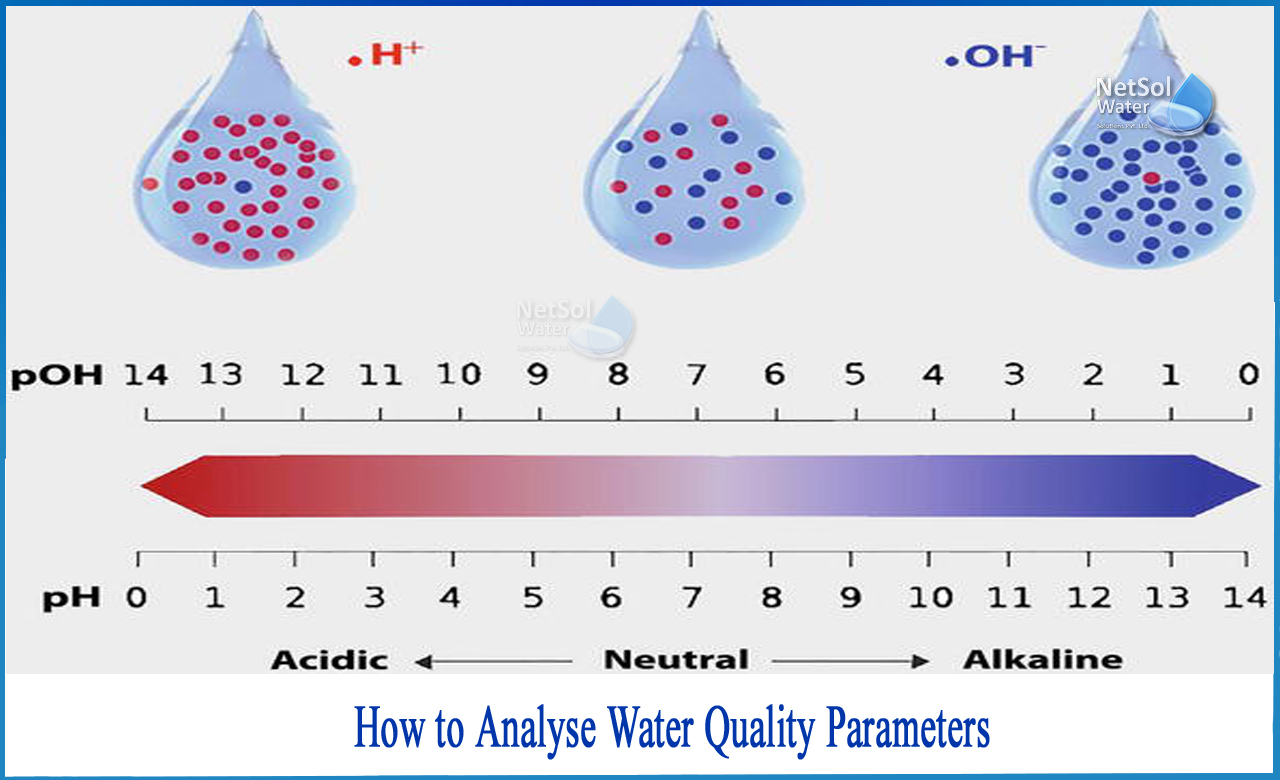
How to analyse water quality parameters?
1: Scavengers of hydroxyl radicals: Hydroxyl radicals (OH) are the driving force behind the advanced oxidation process, and they must be produced and maintained at levels that allow for adequate treatment. Some chemicals, on the other hand, have a high affinity for OH and will react with it before the target pollutants. This lowers the rate at which the treatment is completed.
2: Bromide: Bromides are particularly problematic in ozone-based advanced oxidation systems. They have the ability to produce bromate (BrO3–) in the presence of ozone, in addition to being OH scavengers. Bromate is a carcinogen that has been linked to cancer. To avoid this, either something needs to be done to the bromide ions prior to oxidation, or additional chemicals must be added to discourage bromate production.
3: Organic Matter in Its Natural State: The term "natural organic matter" refers to a wide variety of dissolved organic compounds found in wastewater. They react with OH, just like other scavengers, and limit the system's reaction rate by using up the radicals needed to oxidise the target pollutants. They can, however, operate as UV light blockers, reducing the oxidising agent's capacity to react with it and preventing the appropriate production of OH radicals.
4: Carbonates/Bicarbonates: These molecules react with OH radicals to produce further oxidising agents, resulting in a chain of oxidation processes that slow down the advanced oxidation process' reaction rate and efficiency.
5: Nitrite/nitrate: In the presence of UV radiation, nitrate (NO3–) can be transformed to nitrite (NO2–). As a result, in the case of advanced oxidation, the absorption of radiation by nitrate can obstruct UV processes. As a result, its availability to react with the oxidising agent is reduced. Nitrates, when reduced to nitrite, become an OH scavenger, lowering the reaction efficiency and removal rate of the advanced oxidation systems.
6: pH: The overall concentration of the OH radical is determined by how acidic or basic the effluent is. There is an enhanced presence of a few specific chemicals that affect the generation and utilisation of hydroxyl radicals at various pH levels. Carbonates and bicarbonates, which are hydroxyl radical scavengers, are found in greater abundance at higher pH values.At higher pH levels, the presence of hydroxide and hydrogen peroxide ions is likewise higher. In both ozone and peroxide-based solutions, they can boost the generation of OH.
7: Temperature: There are two aspects to the effect of temperature on advanced oxidation systems. On the one hand, if ozone is used as an oxidising agent, greater temperatures reduce its solubility. Increased temperature, on the other hand, accelerates reaction rates.
8: Solids: The turbidity of the effluent in advanced oxidation systems could be higher than expected if primary and secondary procedures are ineffective. The oxidising chemicals' ability to absorb UV radiation is hampered with higher degrees of turbidity.
Conclusion
Some of these water quality measures have a direct impact on the existence and response of others. As a result, modifying effluent characteristics to improve reactivity and efficiency while also adjusting them to reduce undesirable elements is frequently a balancing act.
Changing the pH or temperature can help with some of the hydroxyl scavengers, but this can reduce the effectiveness of the process. Other chemicals can be introduced to intercept them before they come into touch with the OH radical, slowing down their reaction speeds.
When it comes to selecting advanced oxidation systems based on water quality parameters, there are a lot of intricacies and limitations to consider, and it can be confusing at first.This is an instance where prior expertise is advantageous. With the help of an experienced water treatment engineer on your side, you can make a far better selection.