Explore Effective Electrochemical Oxidation for Pharmaceutical Wastewater Treatment. Compact, Cost-Efficient
Pharmaceutical industry is an important for generating medicines that help us in maintaining our good health, but they release some toxic pollutants during their manufacturing processes that, if left unchecked, can be a serious threat to both humans and the environment. Therefore, to control this deadly disaster various treatment methods are employed so that the effluent released by them gets proper treatment before they are finally disposed off. Among different methods, a well-known process that has shown promising results in cleaning up the wastewater is Electrochemical oxidation. In this blog, we will discuss about it in detail from the process to its advantages.
Let us Understand Electrochemical Oxidation?
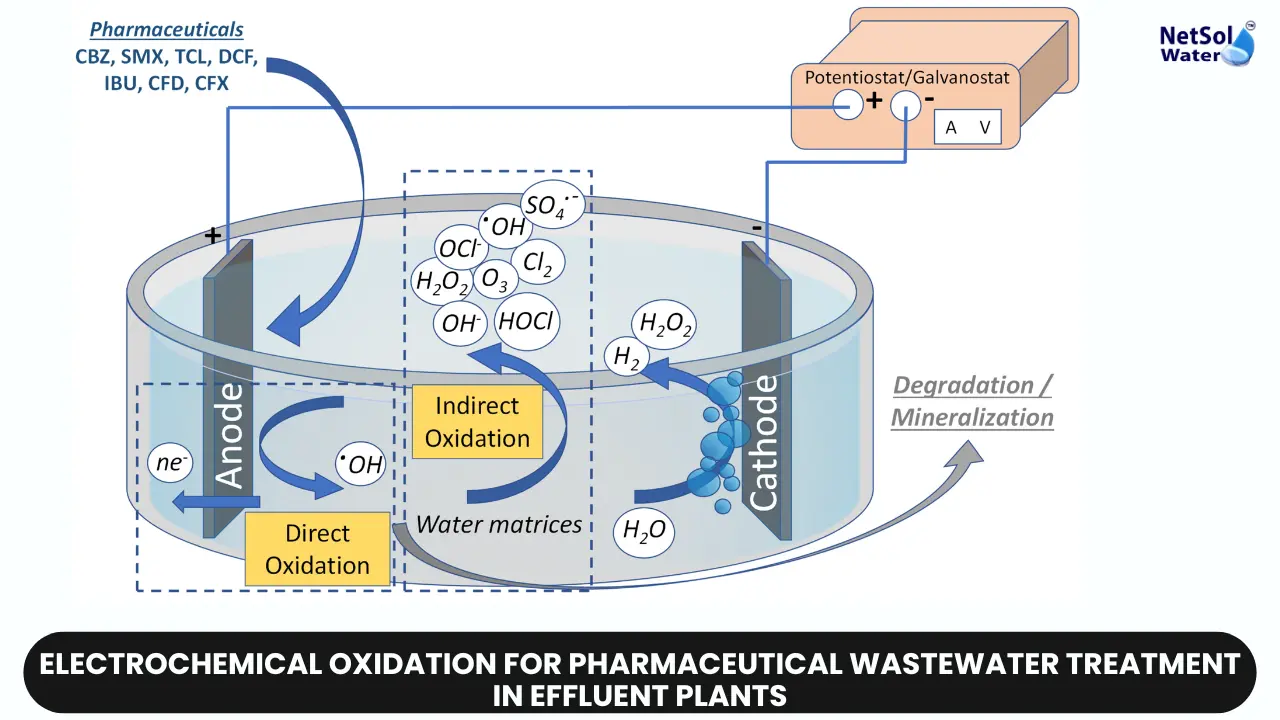
Electrochemical oxidation (EO) is an advanced method for breaking down organic pollutants using electricity to create powerful oxidizing substances like hydroxyl radicals (•OH). These radicals react with pollutants at the anode surface, breaking them down into harmless substances or completely mineralizing them.
When compared to different traditional treatment methods, EO has numerous benefits. Most importantly, it degrades the tough pollutants effectively that are hard to remove using other methods. Next, it doesn't require the addition of extra chemicals, making it more environmentally friendly. Additionally, EO systems are designed to be compact and modular, allowing for flexible installation. Furthermore, they come with relatively low operational costs, making them cost-effective solutions for wastewater treatment. Finally, EO systems can potentially be set up directly at the wastewater source, offering on-site treatment options
How Electrochemical Oxidation Works?
The electrochemical oxidation process follows several key steps. Firstly, an electrolytic cell is set up with an anode and a cathode, immersed in the wastewater to be treated. The choice of anode material, such as boron-doped diamond or mixed metal oxides, is important for the process's efficiency.
Once the cell is operational and an electric potential is applied, oxidation reactions occur at the anode, producing reactive oxygen species like hydroxyl radicals. These radicals then react with and break down the organic pollutants present in the wastewater.
Simultaneously, reduction reactions take place at the cathode, often resulting in the formation of hydrogen gas or the reduction of dissolved oxygen.
The treated wastewater, now containing reduced levels of organic pollutants, is collected. Depending on the requirements, it may undergo additional polishing steps before being discharged into the environment or reused.
Applications in Pharmaceutical Wastewater Treatment
Electrochemical oxidation has proven to be effective in treating various types of pharmaceutical wastewater, including:
1. Antibiotic wastewater: EO can effectively degrade antibiotic compounds, such as penicillins, cephalosporins, and fluoroquinolones, often resistant to conventional treatment methods.
2. Analgesic and anti-inflammatory drug wastewater: Compounds like acetaminophen, ibuprofen, and diclofenac can be effectively degraded using EO.
3. Cytostatic drug wastewater: EO has shown excellent results in the treatment of wastewater containing cytostatic drugs, which are commonly used in cancer treatment and can be highly toxic to aquatic life.
4. Hormone and steroid wastewater: Hormones and steroids, which can disrupt endocrine systems in aquatic organisms, can be effectively removed using EO.
Factors Influencing Electrochemical Oxidation Efficiency
The efficiency of the electrochemical oxidation process is influenced by various factors, including:
1. Anode material: The choice of anode material significantly impacts the generation of reactive oxygen species and the overall oxidation potential. BDD anodes are known for their high oxidation power and stability.
2. Current density: Higher current densities generally lead to increased generation of ROS and faster degradation rates, but excessive current densities can also result in energy inefficiencies.
3. pH and temperature: The pH and temperature of the wastewater can also affect the oxidation mechanisms and the stability of the reactive species generated.
4. Electrolyte composition: Certain ions or compounds in the wastewater can influence the electrochemical reactions and potentially foul the electrode surfaces.
5. Reactor design: The design of the electrochemical reactor, including the electrode configuration and flow patterns, can impact the mass transfer and overall treatment efficiency.
Conclusion
Electrochemical oxidation (EO) is a great way to clean up wastewater from pharmaceuticals. It uses electricity and special chemicals to break down stubborn pollutants in a small, cost-effective setup. EO can handle lots of different types of drugs in the wastewater, like antibiotics and painkillers.
With the pharmaceutical industry growing, it's crucial to use advanced methods like EO to treat wastewater responsibly and protect the environment. By keeping up research on EO and making sure there are rules in place, we can make the pharmaceutical industry cleaner and more sustainable.
Do you need an advice or assistance on selecting the best water and waste water treatment unit? We have solutions for all your problems!
Let us now your problem, our experts will make sure that it goes away.
For an assistance or related query,
Call on +91-965-060-8473
Or write us at enquiry@netsolwater.com