What is the process of Coagulation and flocculation in ETP Plant?
Both the treatment of wastewater and drinking water must include coagulation and flocculation. In the process of treating water, suspended solids are removed using flocculation and coagulation, which destabilises the suspended particles in water solutions.
The distinction between the two is that flocculation is the settling of coagulated particles, whereas coagulation is the joining together, or clumping of particles. The larger aggregated particles quickly separate from the water, and settle due to their ability to easily remove and neutralise charge density of particles, and then enhance particle binding with flocculation.
In this article, we will look at the process of coagulation and flocculation in ETPs. We will also see how coagulants form flocs in the process of wastewater treatment.
History of use of coagulants in wastewater treatment
Up until the invention of the trickling filter for biological treatment in the late 19th century, physical-chemical treatment of wastewater was an extensively used method. There was a brief resurgence of interest in the early 1970s, which has persisted to the present day particularly for treatment plants, which are overburdened during peak flow periods.
The strain on downstream biological processes, or in some situations for direct discharge, can be effectively reduced by adding coagulant chemicals to primary clarifiers, or other specific physical separation processes. It is known as chemically enhanced primary treatment, or CEPT.
What are coagulants?
The two main types of regularly used metal coagulants are those based on aluminium and those based on iron.
1: Aluminium sulphate, aluminium chloride, and sodium aluminate are some of the aluminium coagulants.
2: Ferric sulphate, ferrous sulphate, ferric chloride, and ferric chloride sulphate are the iron coagulants.
3: Hydrated lime and magnesium carbonate are also utilised as coagulants.
Why are aluminium and iron coagulants useful?
The main reason why aluminium and iron coagulants are useful is that, they can create multi-charged poly-nuclear complexes with improved adsorption properties. The pH of the system may be able to influence the type of complexes that form.
The metal ions (Al and Fe) hydrolyse quickly but unpredictably when metal coagulants are introduced to water, creating a variety of metal hydrolysis species. Which hydrolysis species is suitable for treatment, depends on the effectiveness of quick mixing, pH, and coagulant dosage.
Pre-hydrolysed inorganic coagulants
Pre-hydrolysed inorganic coagulants based on both aluminium and iron has undergone significant developments, in order to produce the proper hydrolysis species regardless of the treatment process circumstances. They include types of Polyaluminum chloride containing organic polymers, Polyaluminum sulphate chloride, Polyaluminum silicate chloride, and aluminium chlorohydrate. Ferric salts containing polymers and polyferric sulphate are examples of iron formations.
Benefits of pre-polymeric inorganic coagulants
The ability of pre-polymerized inorganic coagulants to act well, over a wide range of pH and raw water temperatures is one of their main benefits. They require lower doses to meet water treatment goals, are less sensitive to low water temperatures, produce less chemical residues, and produce less chloride or sulphate residuals, which lowers the TDS of the final water. Moreover, they generate fewer metal residues.
Bridging mechanism of polymers
Electrostatic and bridging effects are two significant mechanisms affecting polymer, while they are being treated. When the charge density of a polymer increases for a given molecular weight, the polymer chains are stretched due to an increase in electrostatic repulsion between charged units, which raises the viscosity of the polymer solution.
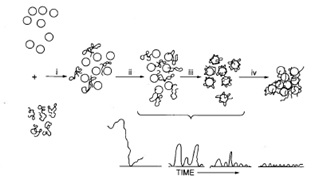
Stages in the bridging mechanism: Dispersion (ii) Adsorption (iii) Compression or settling down (iv) Collision
Limitation of synthetic polymers used for coagulation process
One issue with synthetic polymers is potential toxicity problems, which typically result from leftover unreacted monomers. The fraction of unreacted monomers may be managed during production, and treated water typically will contain only small amounts of them.
Variables influencing coagulation process
· Temperature
By altering the optimal pH, temperature has a considerable impact on coagulation processes, especially in low turbidity fluids, operating at the optimal pOH, which is determined by:
pH + pOH = p KW
pKW = 0.01706xT + 4470.99/T - 6.0875
Where, T = temperature in degrees
Kelvin = 273.15 + °C.
· Chemical addition
It is customary to add chemicals for pH correction first, then the metal coagulant, and finally the flocculant aid when performing coagulation procedures. Not all of these substances must be introduced, but the indicated sequence must be followed.
Other sequences, such as inverting the order of metal coagulant and polymer addition, and the order of metal coagulant addition and pH adjustment, can, nevertheless, be more productive in some circumstances.
· Residual aluminium
The presence of residual aluminium in treated water is not only unsightly, but it may also have negative neurological effects like Alzheimer's disease. Even though, drinking water consumption only accounts for a small fraction of daily intake, residual aluminium in treated waters can be reduced with the right pH adjustments.
What is flocculation?
Flocculation is the process by which particles join together and bond with one another to produce big, agglomerated particles that are easily settled when their interactions in water are destabilised, in the presence of another molecule.
Because, their surface charges can be adjusted to change their destabilising potential, polymeric molecules are frequently utilized to aid this process. The potential for destabilising effects increases with charge density. The less impact they have on the particles in suspension, the lower the charge density.
What is orthokinetic flocculation?
Orthokinetic flocculation results from created liquid velocity gradients. It is at this point that primary particles are propelled to reach to one another to touch, and gradually form larger agglomerates or flocs.
The applied velocity gradient is the main factor affecting the rate of orthokinetic flocculation. Both the applied velocity gradients and the flocculation period determine the extent or degree of flocculation. The rate and degree of particle aggregation as well as the rate and degree of breakdown of these aggregates, are influenced by these two characteristics.
Baffled chambers, granular media beds, diffused air, spiral flow chambers, reciprocating blades, and rotating blades, are some of the methods used to create velocity gradients.
Factors affecting the coagulation-flocculation process
Several factors affect how effective the coagulation-flocculation process is. For a specific body of water, these could include:
· Utilized coagulant type
· Dose of a coagulant
· pH
· Concentration of coagulant feed
· Chemical additions other than the principal coagulant, their type, and dosage (e.g. polymers)
· Chemical addition in order and the interval between dosage locations
· Mixing intensity and time at the quick mix stage
· Rapid mix device type
· Use of velocity gradients during the flocculation stage
· Time for flocculator retention
· Utilized stirring method
· Geometry of the flocculator
How do coagulants form flocs in the process of wastewater treatment?
1: When a colloidal suspension coagulates or destabilises, tiny particles are joined by physical and chemical processes.
2: Through bridging, flocculation causes the creation of a larger settleable structure. These procedures have been widely utilised to get rid of colour or suspended particles.
3: Adsorption of ionic forms also occurs, however to a lesser or greater amount, primarily dependent on the components of the water or wastewater.
What are the three destabilising mechanisms for colloids?
Destabilization of coagulants in wastewater treatment occurs as follows:
1: Drainage (creaming),
2: Coalescence (film breakage),
3: Ostwald ripening (disproportionation), and
4: Sedimentation.
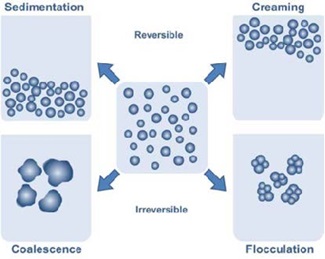
Destabilisation of coagulants during the quick or rapid mixing
Given that destabilisation reactions take place during the rapid mixing stage, and that this is also where primary floc particles are formed (whose properties have a significant impact on subsequent flocculation kinetics), it is possible that this stage is the most crucial in coagulation-flocculation processes.
The most significant for successful destabilisation are most likely the metal coagulant hydrolysis products, which are generated between 0.01 and 1.0 seconds.
Traditional quick mixing retention lengths of 30 to 60 seconds are frequently not sufficient, and flocculation effectiveness could not increase over rapid mixing times of 5 seconds or less. In fact, after a certain maximum quick mix time, flocculation effectiveness may suffer.
Coagulation-flocculation in stages can improve CEPT performance!
For instance, TSS and BOD reductions of 80 to 95% and 58 to 68%, respectively, can be achieved using 60 mg/l ferric chloride, followed by 15 mg/l Polyaluminum chloride, followed by 0.5 mg/l anionic polymer, at main clarifier overflow rates of over 6 m/h (3,600 gpd/ft2) during peak flow treatment. At peak flow, the reaction can take around 8 minutes to complete from the point of ferric chloride addition, to when it entered the primary clarifiers.
Conclusion
In large urban areas that have developed sewerage systems but without centralised wastewater treatment, and have insufficient financial resources for more comprehensive, but capital intensive biological treatment options like activated sludge systems, CEPT can also be an efficient first step for pollution control in developing countries, like India.
Also, the effectiveness of CEPT for removing BOD or COD relies on the properties of the wastewater. One can anticipate that CEPT will remove some of the colloidal components as well as the particle components. Consequently, it is possible to remove more than 95 percent TSS, 65 percent COD, 50 percent BOD, 20 percent nitrogen, and 95 percent phosphorus from such effluent.
In reality, removals may be higher or lower. One may anticipate fewer overall removals using CEPT in warmer regions with larger collecting systems, and generally level sewers due to a higher degree of hydrolysis of particulate matter. On the other hand, there can be a bigger risk if the collection system is modest, the weather is chilly, and the wastewater is still quite fresh.
Leading manufacturer and supplier of wastewater treatment plants in India
Netsol Water is the leading manufacturer, supplier, and exporter of a quality selection of water treatment and wastewater treatment products in India.
Reverse osmosis plants as well as water softeners, ETPs, DM plants, AMC, O&M, Ultra filtration, UV water purification systems, STPs, ZLD plants, and other goods and services are available from us. We also provide services to businesses including automotive, pulp & paper, beverages, pharmaceutical, textile, refineries, schools, hotels, hospitals, office buildings, among others.
Call us at +91 9650608473 or email at enquiry@netsolwater.com for further information.



