Ionexchange resins are constructed of a porous, polymeric substance that is insoluble in acid, base, and water. These resins are made up of small hydrocarbon beads that are roughly 12 millimetres in diameter.
Anion and cation resins are the two most common forms of ion exchange resins. Ion exchange resins are classified into four categories such as
- 1. Strong Base Anion
- 2. Weak Base Anion
- 3. Weak Acid Cation
- 4. Strong Acid Cation
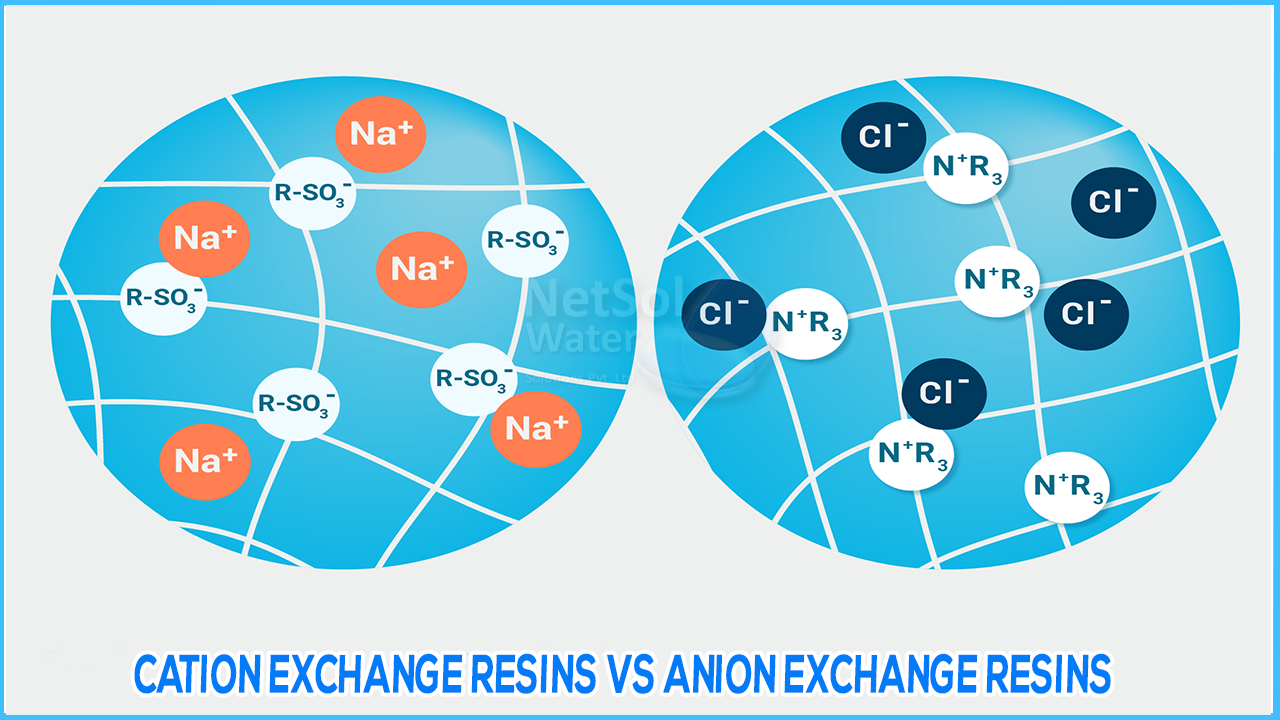
Difference between anion exchange resin and cation exchange resin
The two most frequent resins used in the ion exchange process are anion and cation resins. Anion and cation resins vary in a way that one is positively charged (cation) and the other is negatively charged (anion)
Positively charged resins attract negatively charged ions, whereas cation resins attract negatively charged ions. When choosing an anion vs. cation resin, there are a variety of physical and chemical features to consider that can help you choose the optimal resin for your purposes, such as:
- 1. The size of the resin beds.
- 2. The amount of water the resin can hold.
- 3. The amount of ions the resin can filter out before needing to be regenerated.
- 4. The rate at which water can pass through the ion exchange resin.
- 5. The desired final effluent water quality.
- Contaminants in the source water, including their type and amount.
In today's industrial water treatment systems, cation and anion exchange resins are frequent and necessary components. Many dissolved minerals in source water must be eliminated to avoid wreaking havoc on sensitive industrial systems. Small concentrations of these minerals can contaminate delicate electrical components and decrease the performance of industrial equipment.
Cation and anion exchange resins can be thought of as powerful magnets that use electromagnetic attraction to attract and hold contaminated minerals from the passing source water. Cation resins inherently attract one set of minerals, whereas anion resins naturally attract a different set.
The surface area of the polymeric resin beds is increased by treating them with thousands of microscopic fractures. Mineral-laden molecules "cling" to the numerous surfaces of the cation and anion resin beds as source water travels through them. This process continues until all of the beds are covered in minerals, at which point the resin must be regenerated to remove the minerals and restore the resin to its original state.
Anion vs Cation-De-alkalization
For demineralization or de-alkalization, strong base anion resins are excellent, while weak base anion resins are best for removing acids and organics from water. With a low-to-moderate level of total dissolved solids, anions are generally utilised to reduce alkalinity from boiler feedwater (TDS). Carbonate, bicarbonate, sulphates, and nitrates can all be removed from feedwater using anions.
Demineralization or de-alkalization (most typically in hydrogen form) or split stream de-alkalization can both be accomplished with strong acid cation resins. Both de-alkalization procedures, however, necessitate the use of hazardous acids to regenerate resin beds, as well as the usage of a de-gassifier to remove carbon dioxide produced during the treatment process. Weak acid cation resins can also be used for demineralization and de-alkalization. In most water treatment cases, weak acid cation resins are used to remove divalent ions associated with alkalinity. Weak acid cation resins work best when water has a hardness and alkalinity ratio of 1:1 or higher.