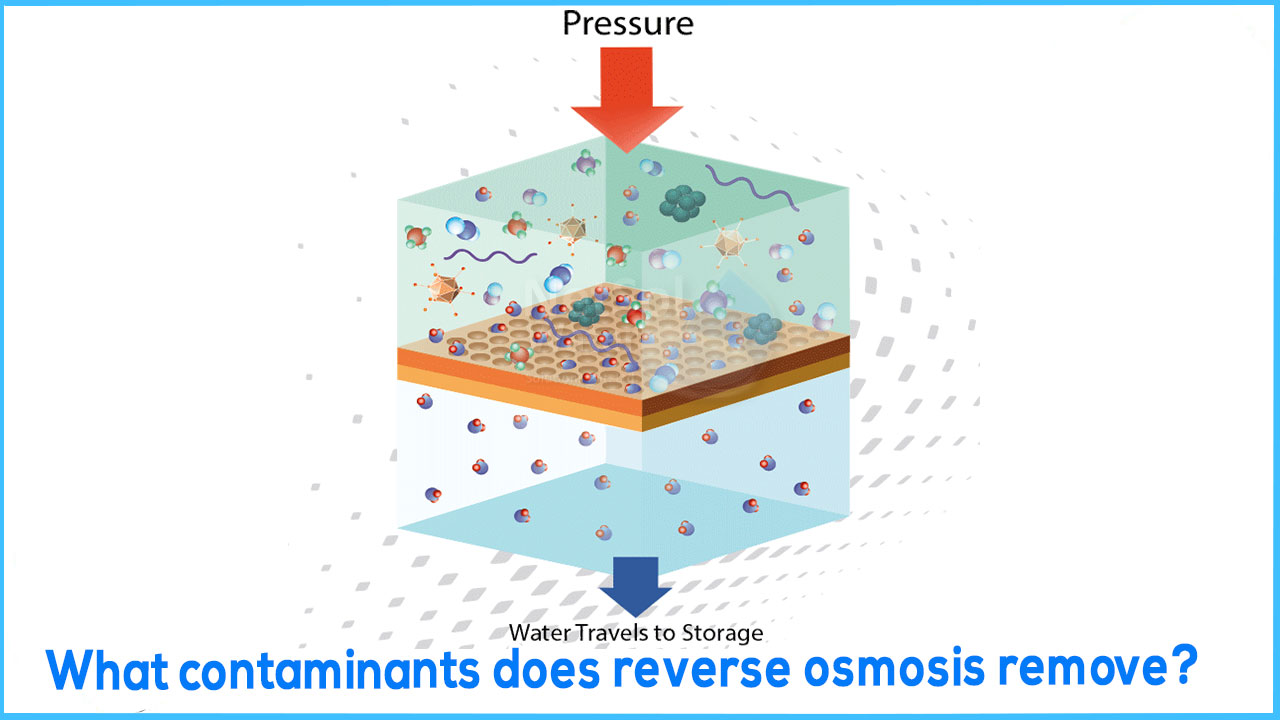
Reverse osmosis (RO) is a membrane diffusion process that is driven by pressure. In practise, RO membranes retain 95–99 percent of dissolved solutes (organic and inorganic) in the concentrate, while the permeate is high-quality water. As a result, RO is considered a concentration procedure.When compared to other concentration processes, RO has various advantages. The elimination of the solvent does not necessitate a phase transition, hence RO saves energy. In comparison to other competitive technologies, RO is more cost-effective when it comes to concentrating diluted solutions and medium concentrations. Furthermore, unlike other concentration methods, RO concentrated fluids are not vulnerable to heat degradation or aroma compound losses.
Water pressure is used to force water through a semi-permeable membrane in the reverse osmosis process. When water is pressed against the reverse osmosis membrane surface, dissolved materials are rejected while water molecules diffuse molecule by molecule through the membrane, resulting in purer water on the other side. This technology of "reverse osmosis" is widely used to reduce pollutants in water. Contaminants removed by a typical RO system is represented in tabular form as:

Other TDS contaminants reduced by up to 98%: Aluminum, Ammonium, Bicarbonate, Calcium, Chlorine, Chromate, Cyanide, Ferro cyanide, Iron, Magnesium, Mercury, Manganese, Phosphate, Silicate, Silver, Sodium, Strontium, Sulfate, Sulfite, Thiosulfate, Zinc.
Reverse osmosis (RO) does not guarantee that all contaminants will be removed completely. It may be discovered during onsite water analysis that a pollutant identified in the feed water is not detectable in the RO water. This conclusion, however, may change with each subsequent analysis. The most important thing to remember from reading a membrane spec sheet is that RO rejects a certain percentage of pollutants, which changes depending on the contaminants and conditions.
Furthermore, different membranes have distinct rejection properties.From a technological standpoint, an RO membrane will reject any material with a molecular weight greater than 100. In general, RO membranes remove between 95 and 99 percent of inorganic particles. Calcium, Strontium, Copper, and compounds like Sodium Chloride are examples of this. At maximal efficacy, organic material such as bacteria and viruses are eliminated at a rate of 99.9%. The water generated may not always be devoid of bacteria and viruses.While the RO membrane removes chlorine compounds, it is hydrolysed and destroyed by chlorine. The pace of destruction is determined by the amount of chlorine in the water. It is recommended that a RO membrane be pre-treated with Activated Carbon to remove chlorine, a water softener to remove hardness that will foul the membrane, and sediment filtering to prevent the membrane from plugging.
Dirt, hardness, algae, and mould are all removed by RO, but these should be filtered out to avoid premature membrane failure.The overall rejection % will not be affected if one or two membrane fibres rupture. Because bacteria and viruses are living, replicating creatures, even a little drop in quantifiable rejection can result in an outbreak of infectious growth. Also note that your results may vary depending on how well you maintain your unit, how well it functions, and how much water is available at the start.