What is the use of water softener in electroplating?
Italian inventor Luigi V. Brugnatelli invented electroplating in 1805. He accomplished this by connecting a wire between a dissolved gold solution and a battery, also known as a Voltaic pile. A metal object was then connected to the wire, allowing the gold to become attached to it.
Electroplating is the method of plating one metal onto another through hydrolysis, usually to prevent metal corrosion or for decorative purposes. The process employs an electric current to minimise dissolved metal cations in order to form a lean coherent metal coating on the electrode. Electroplating is frequently used in the electrical oxidation of anions on a solid substrate, such as in the formation of silver chloride on silver wire to form silver chloride electrodes.
Electroplating is most commonly used to modify the surface properties of an object (e.g., corrosion protection, lubricity, abrasion), but it can also be used to build thickness or make objects by electro forming.
THE ANODE AND CATHODE
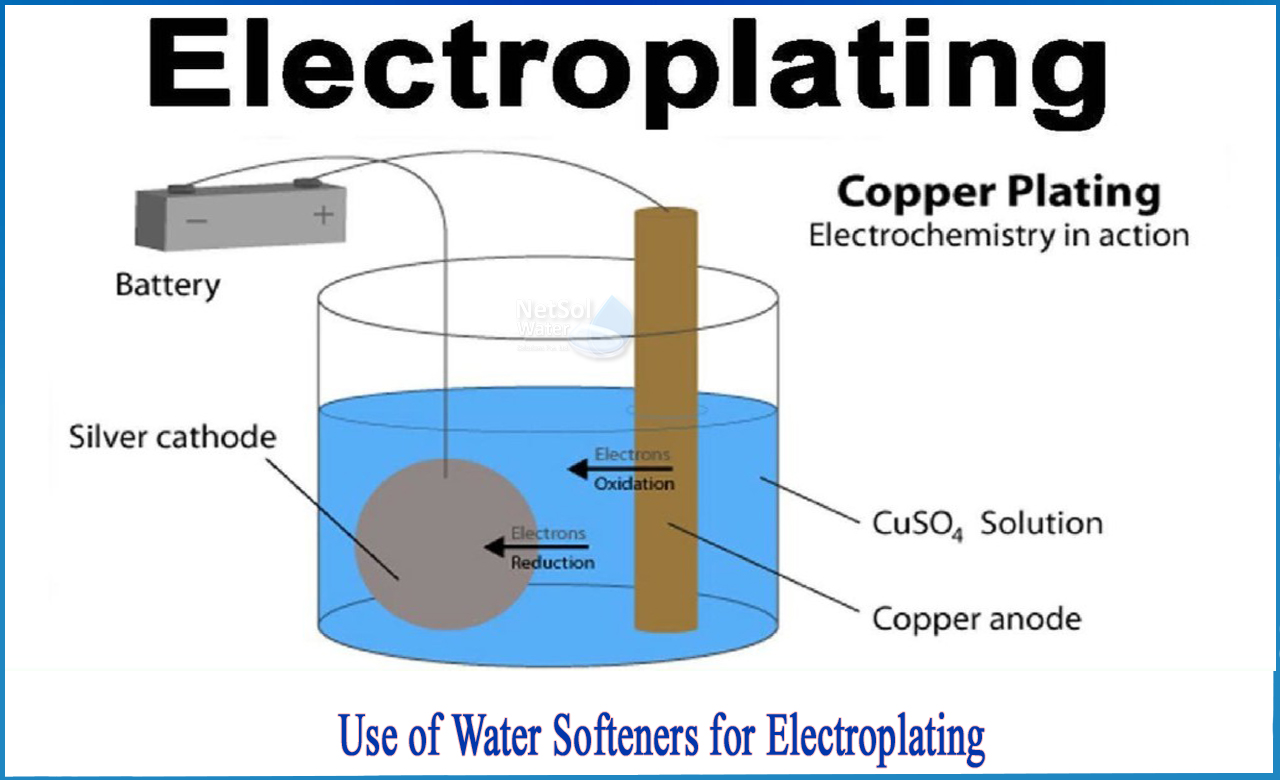
A water solvent is used to create an electrolyte solution; in some cases, an organic or ionic solvent may be used. Two electrodes are necessary for electroplating: a cathode (working electrode) and an anode (counterelectrode).
In electroplating, current is usually presented from outside, and the anode is really the positive electrode and the cathode is the negative electrode. The cathode is the electrode that undergoes the electrochemical reduction reaction. The anode is the spot of the electrochemical oxidation reaction.
An anode and a cathode are used in the electroplating process. The metal dissolved from the anode can be plated onto the cathode in electroplating. The anode receives direct current, which oxidises and dissolves the metal atoms in the electrolyte solution. The dissolved metal ions are reduced at the cathode, and the metal is placed on the product.
ELECTROPLATING AND ELECTROLESS-PLATING
Electroless plating, also defined as chemical or auto-catalytic plating, is a non-galvanic plating technique that requires several concurrent reactions in an aqueous solution that do not require external electrical power. It differs from electroplating primarily in that it does not require external electrical power.
Plating an object strengthens it and increases its corrosion resistance. Electroplating and electro lessplating can be used to coat materials that will be used in the medical field with a protective coating. If the external power source is sufficient, electroplating is quick and effective. This method yields a coat that is smooth and thick enough to protect the material from damage.
WHERE IS ELECTRO PLATING USED?
Cookware, kitchen appliances, pots and pans, and sink taps are just a few electroplating examples that we encounter and use on a daily basis. Silverware cutlery, for example, is electroplated to help retain its appearance and prevent tarnishing.
NETSOL WATER SOFTENER FOR ELECTROPLATING
We, Netsol water, are always ranked amongst the top companies serving electroplating with superior softener plants as then our daily used items can be made correctly, and such units and our softener plants have won profound respect across the country for their high performance and efficiency.
With our water softener plants in place, you can easily eliminate the negative elements that would otherwise remain prominent and cause the water to be hard when our ion exchange process is used.
Netsol Water is Greater Noida-based leading water & wastewater treatment plant manufacturer. We are industry's most demanding company based on client review and work quality. We are known as best commercial RO plant manufacturer, industrial RO plant manufacturer, sewage treatment plant manufacturer, Water Softener Plant Manufacturers and effluent treatment plant manufacturers. Apart from this 24x7 customer support is our USP. Call on +91-9650608473, or write us at enquiry@netsolwater.com for any support, inquiry or product-purchase related query.