How do you remove lead from Industrial waste water?
The removal of (potentially) toxic heavy metal ions from sewage, particularly in industrial and mining waste effluents, has received a lot of attention in recent years. Heavy metal contaminations may exist in waste from a variety of industries, including metal plating, mining operations, tanneries, chloralkaline, radiator manufacturing, smelting and alloy industries, and storage battery industries.
Lead (Pb) is one of the most well-known and closely monitored contaminants in drinking water, but it is also a concern for industrial facilities. These facilities typically pursue lead-removal technologies in order to meet regulatory wastewater contaminant limits, though many of the same technologies can also be used to help meet process water quality requirements or as part of a reuse and reclamation strategy.
To remove lead from industrial water and wastewater, several separation and treatment technologies are commonly used. Each treatment strategy provides specific benefits or fitness for specific applications; thus, the best lead removal technology is one that fits a facility's specific needs.
The following is a brief overview of water and wastewater treatment technologies that are commonly used for lead removal:
1-Precipitation by chemical means
Chemical precipitation is one of the most commonly used technologies for removing lead from water and wastewater streams. It is a relatively simple and inexpensive process that is effective for reducing dissolved lead content in streams with high concentrations of the metal.Chemical precipitation works on the premise that the solubility of lead (and other heavy metals) is proportional to the metal concentration and the pH of the solution. The heavy metal precipitation process begins with the addition of a caustic chemical to the wastewater stream, such as hydroxide, to raise the pH to the point where the dissolved lead precipitates from the solution as solid metal hydroxide particles. The precipitated lead particles are then removed from the liquid stream using a physical separation method such as sedimentation or media filtration.
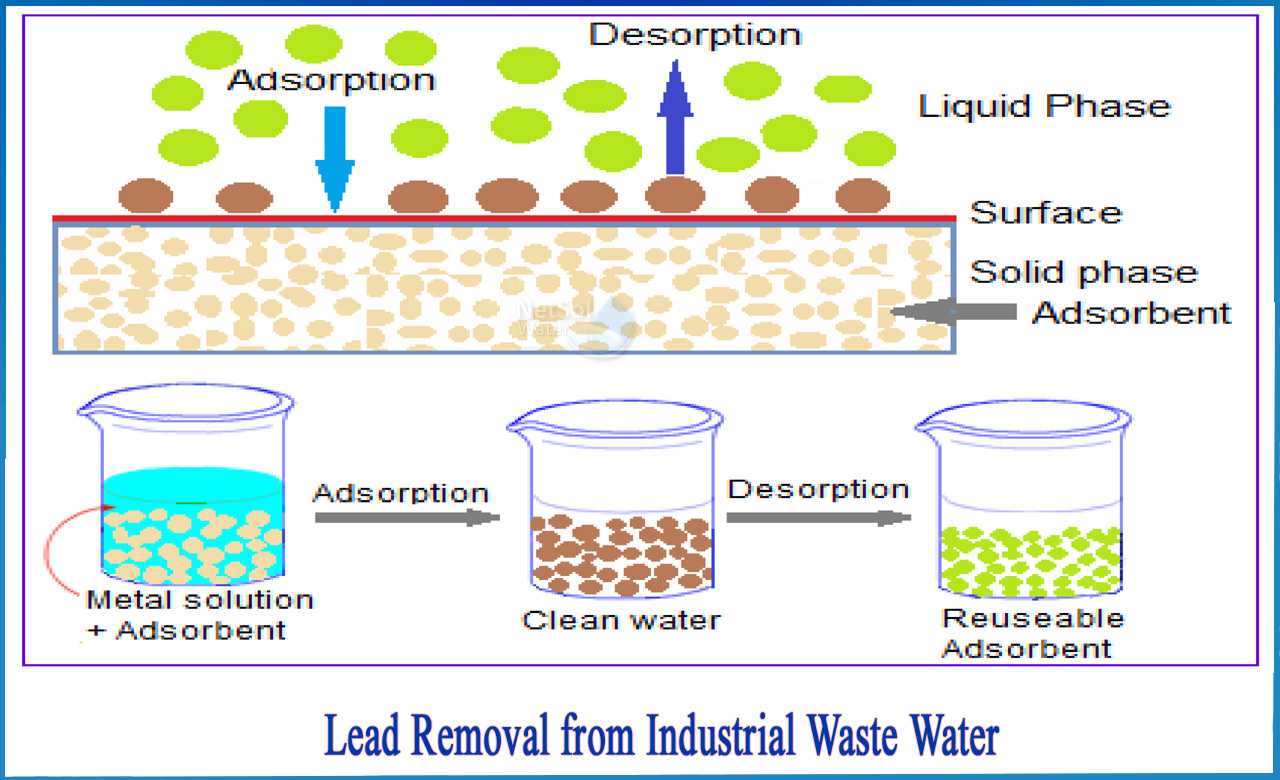
2-Adsorption
Adsorption, along with chemical precipitation, is one of the most commonly used technologies in lead removal applications. Adsorption is a low-cost method of treating streams with moderate lead concentrations (usually between 1 ppb and 100 ppm). Adsorption has several advantages, including no sludge generation, a wide range of affordable adsorbent materials, and good selectivity for heavy metals.
Adsorption is a process that separates contaminants from water by using molecular forces of attraction. Adsorbent media are carefully chosen for their ability to draw a specific dissolved material (or "solute") out of solution, effectively holding the solute to the adsorbent media's surface as a molecular or atomic film known as an absorbate. For lead separation, physical adsorption technologies are commonly used, and the process involves passing a liquid stream through some type of granular or porous adsorbent media.For lead-removal applications, a variety of adsorbent media are available, including activated carbon, zeolite, and biomass.
3-Ion exchange
Ion exchange (IX) is effective for reducing lead content below stringent regulatory limits, as well as for applications requiring selective lead removal. Furthermore, IX is capable of efficiently treating large volumes of water.
IX is a physical-chemical process that involves passing a liquid stream through one or more columns containing a resin substrate. The resin has an ionic charge that is used to separate specific contaminants from solution. Facilities use either a strong acid cation (SAC) or a weak acid cation (WAC) resin for lead removal applications. As a lead-contaminated stream passes through the IX unit, the SAC or WAC resin selectively captures charged lead ions from solution and holds them until the resin goes through a regeneration cycle.There are also specialty resins that focus on specific types of ions and removal to low levels and are used when conventional resins are unable to achieve the treatment goals.
4-Membrane separation
Membrane separation is a class of physical separation technologies that employ a permeable barrier to remove specific constituents from a liquid stream. Membrane separation technologies have gained ground on traditional physical-chemical water treatment technologies in recent decades, owing to advancements in system design and membrane materials. Membrane separation, depending on the type used, is capable of removing up to 90% of lead from water and wastewater streams, can be used for stream constituent reclamation, and is effective in removing hardness, colors, odors, and TDS in addition to heavy metals such as lead.
The membrane separation process entails passing a liquid stream through a porous membrane, usually under pressure. A membrane's pores are specially sized to allow certain particles to pass through while retaining others. In this way, membrane separation works on the principle of size exclusion, and different types of membrane technologies are thus classified based on pore size, with nanofiltration (NF) and reverse osmosis (RO) being the two types of membrane filtration with pores small enough to remove divalent cations, such as lead.
CONCLUSION
Several approaches have recently been studied in order to develop cheaper and more effective technologies, both to reduce the amount of produced wastewater and to improve the quality of the treated effluent. In recent years, adsorption has emerged as an alternative treatment option, and the search for low-cost adsorbents with metal-binding capacities has intensified. Although many techniques can be used to treat heavy metal-contaminated wastewater, it is important to note that the best treatment for metal-contaminated wastewater is determined by some basic parameters such as pH, initial metal concentration, overall treatment performance compared to other technologies, environmental impacts, and economic parameters such as capital investment and operational costs.
For more information, contact Netsol Water.