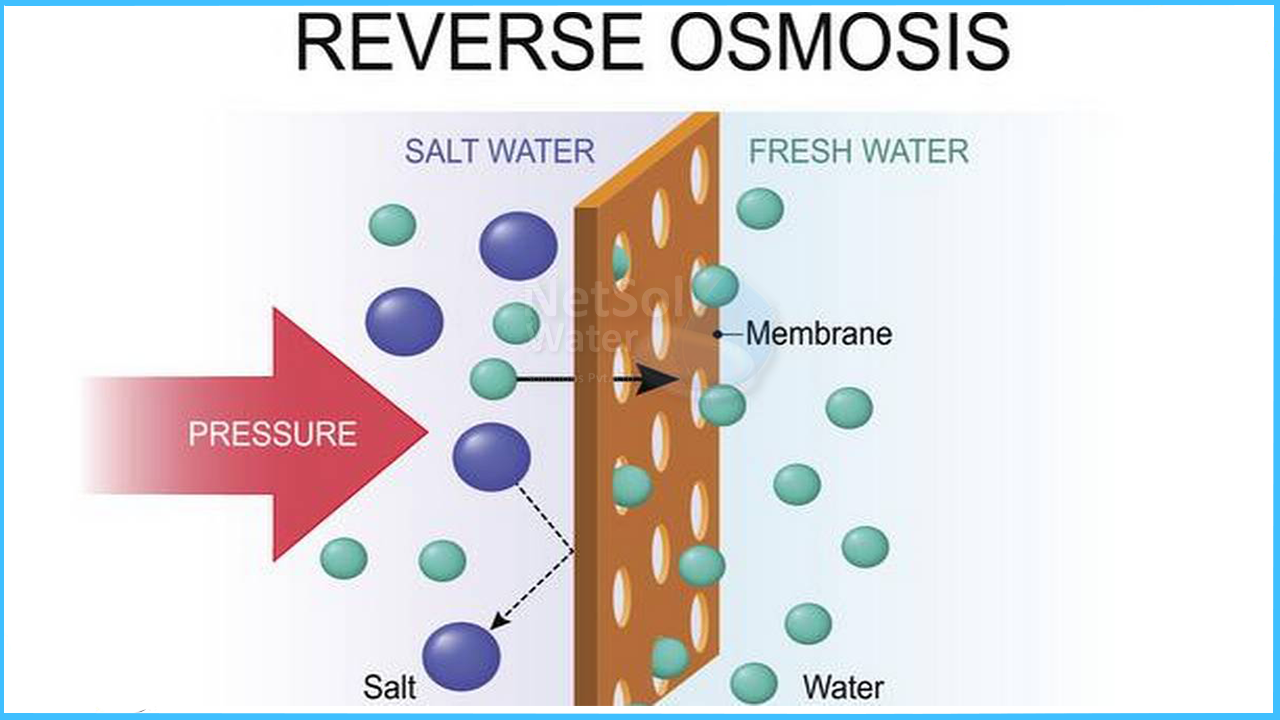
Reverse osmosis (RO) is a membrane diffusion process that is driven by pressure. In practice, RO membranes retain 95–99 percent of dissolved solutes (organic and inorganic) in the concentrate, while permeate is a high-quality water. As a result, RO is considered a concentration procedure.
When compared to other concentration processes, RO has various advantages. The elimination of the solvent does not necessitate a phase transition, hence RO saves energy. In comparison to other competitive technologies, RO is more cost-effective when it comes to concentrating diluted solutions and medium concentrations.Furthermore, unlike other concentration methods, RO concentrated fluids are not vulnerable to heat degradation or aroma compound losses.
When it comes to concentrating liquid foods, this is crucial. RO concentration is best explained as the mechanism of preferential sorption capillary flow, as is the case with other membrane processes. Permeation happens due to the preferential sorption of constituents from a fluid mixture and their permeation through the porous membrane, according to this mechanism. It is critical for RO to occur that a membrane has the right chemical nature (polar and nonpolar effects) as well as the right size and number of holes (steric effect).
The pressure-driven membrane method of reverse osmosis (RO) is used to purify water. Water travels through the membranes more easily than the impurities being removed in all pressure-driven membrane processes. The water supplied to a RO membrane, however, does not always travel through the membrane. The RO process produces two water streams with different water quality:
Stream 1: The cleaned water stream that passes through the membrane is known as "permeate."
Stream 2: The concentration of pollutants on the feed water side increases as the feed water stream goes through the membrane and loses water to the permeate stream. As a result, the feed stream quality degrades as it passes through the membranes, and it is commonly referred to when "concentration" as it exits the membranes.
IS RO SYSTEM DESIGNED FOR BIOLOGICAL PURIFICATION?
The membranes used in RO are typically made up of a non-porous polymeric film with porous support layers underneath. The tiny gaps in this polymeric film provide RO the unique ability to remove the majority of dissolved particles from water. Viruses, proteins, particles,bacteria, salts, heavy metals, dissolved organics, and other pollutants dissolved in water can all be rejected by RO.
RO isn't usually intended for biological water filtration. Minor flaws in the membrane structure may allow microorganisms to slip through without affecting the membrane's capacity to remove dissolved substances. The RO system is unable to remove chlorine from water. Because chlorine can damage the non-porous films that allow dissolved particles to be removed, carbon must be added upstream of the Reverse Osmosis membrane to ensure excellent performance. Although RO is capable of removing some undissolved particles (dirt), it is not designed for this purpose, and utilizing it in this way shortens the membranes' lifespan. As a result, particle filtration before RO is advised.