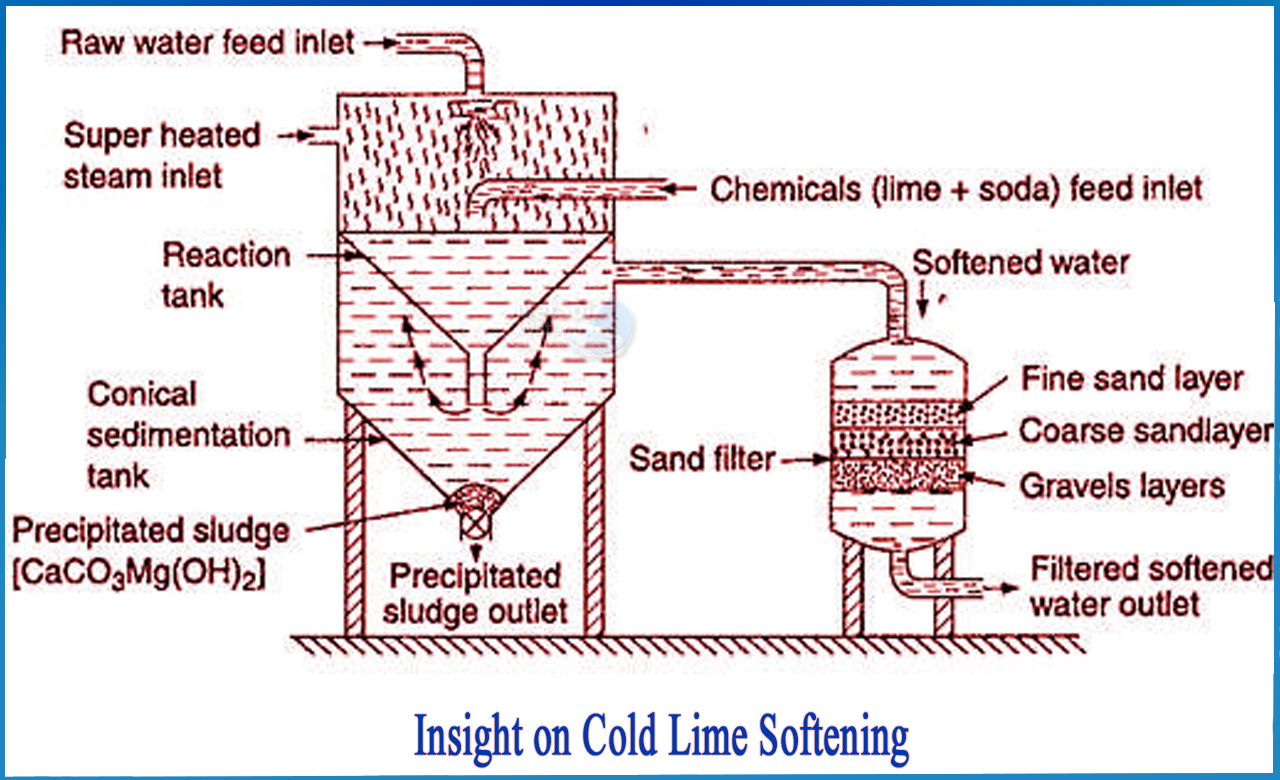
What is cold lime softening?
Cold lime softening, also known as Clark's process, is used to reduce the hardness, alkalinity, silica, and other constituents of raw water. This aids in the preparation of water for direct cooling tower makeup or as a first-stage treatment followed by ion exchange for boiler makeup or RO Reject recycle.
The water is treated with lime or a lime-soda-ash mixture (carbonate ion). These chemicals react with the water's hardness and natural alkalinity to form insoluble compounds. Sedimentation/clarification removes the compounds from the water after they precipitate. Waters with moderate to high hardness and alkalinity levels (150-500 ppm as CaCO3) are frequently treated in this manner.
Cold lime softening is the addition of chemicals to precipitate calcium and magnesium ions. When the mineral content of the source water ranges from 150 ppm to 500 ppm, cold (ambient temperature) lime softening is used. Chemical pre-treatment, clarification, re-carbonation, filtration, and sludge treatment are the five steps in the treatment process.
CHEMICAL COMPOSITION
Hardness is present in almost every raw water supply as calcium and magnesium bicarbonate, also known as carbonate hardness or temporary hardness. These compounds are formed when acidic, carbon dioxide-laden rainwater interacts with naturally occurring minerals in the earth, such as limestone.
CO2 + H2O = H2CO3
H2CO3 + CaCO3 ¯ = Ca(HCO3)2
To kill any living organisms that may be present in the raw water, sodium hypochlorite (bleach) is added. Coagulant (typically ferric or aluminium salts) is sometimes used to help particles bind together. Polymer acts as a glue, gluing individual particles together to form larger clusters. Larger particles settle faster, resulting in a more concentrated sludge.
Both the calcium (and, to a lesser extent, magnesium) in the raw water and the calcium added with the lime are precipitated during the process. In contrast, sodium is exchanged for calcium and magnesium ions in ion exchange softening. Total dissolved solids (TDS) are significantly reduced in lime softening, whereas there is no significant change in TDS in ion exchange softening (also known as zeolite softening).
SOFTENING AND CLARIFICATION
Softening is the process of removing calcium and magnesium from water, which can cause hard water deposits known as "scale." Following the chemical pre-treatment, the water enters the softener. The softener creates a reaction zone for the hardness, which causes ions to precipitate. The lime and (occasionally) soda ash are added just before the feed enters the clarifier in the reaction zone. To remove calcium and magnesium hardness, lime is added to raise the pH.
If the raw water contains enough alkalinity to remove the calcium hardness, soda ash can be added to remove it. Solids that have settled react with lime and soda ash to form larger, faster settling particles. The clarified water is routed through weirs, and the solids (sludge) are scraped to the centre for removal and dewatering. Typically, the overflow contains less than 10 mg/L of suspended solids. The amount of hardness remaining will depend on the water chemistry and proper chemical addition.
Netsol Water is Greater Noida-based leading water & wastewater treatment plant manufacturer. We are industry's most demanding company based on client review and work quality. We are known as best commercial RO plant manufacturers, industrial RO plant manufacturer, sewage treatment plant manufacturer, Water Softener Plant Manufacturers and effluent treatment plant manufacturers. Apart from this 24x7 customer support is our USP. Call on +91-9650608473, or write us at enquiry@netsolwater.com for any support, inquiry or product-purchase related query.