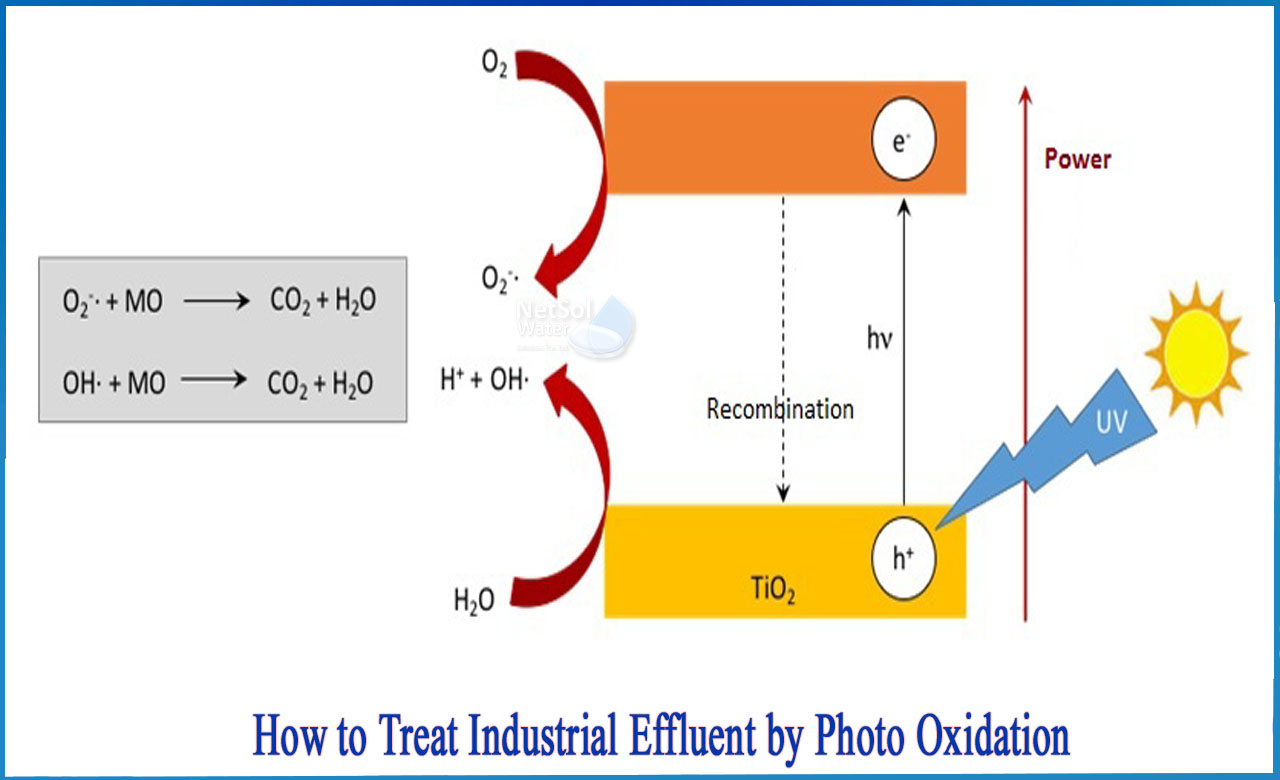
How to treat industrial effluent by photo oxidation?
In cases where conventional processes are ineffective, such as when attempting to decolorize effluent polluted by polyphenolic structures, effluent treatment can be complicated. For these recalcitrant organic products of industrial origin, advanced oxidation processes, also known as photo-oxidation, are a simple and effective solution. Photo-oxidation, in either of its two forms: photolysis or photocatalysis, has one of the best prospects.
What is the goal of oxidation?
The goal of advanced oxidation processes is to generate the hydroxyl radical (OH•), which is extremely reactive due to its high oxidation potential. When these radicals come into contact with organic matter, they set off a chain of chemical reactions that result in the complete mineralization of organic compounds, CO2, and water.
These industrial wastewater treatment processes are very appealing because of the numerous benefits they provide, such as high reactivity with most organic compounds, complete oxidation of both organic and inorganic compounds, and the emission of only harmless compounds, because all oxidants are destroyed in the process.
How does it work?
Photolysis works by exposing the effluent to ultraviolet light (170-230 nm) and allowing the chemical compounds to absorb it and form free radicals. The lower the wavelength of the radiation, the more energy is absorbed and the more efficient the contaminant destruction.
By producing free radicals, radiation causes oxidation reactions. In order for these reactions to take place, oxidizing species must be present in order for these radicals to form. Ozone and hydrogen peroxide are two of the most powerful oxidizing agents. The combination of ultraviolet radiation and ozone or hydrogen peroxide is extremely effective at providing a free radical source for the non-selective oxidation of most organic molecules.
What are advanced oxidation processes?
In a broad sense, advanced oxidation processes (AOPs) are a set of chemical treatment procedures designed to remove organic (and sometimes inorganic) materials in water and wastewater through oxidation via reactions with hydroxyl radicals (OH). However, in real-world wastewater treatment applications, this term usually refers to a subset of such chemical processes that use ozone (O3), hydrogen peroxide (H2O2), and/or UV light. In situ chemical oxidation is one example of such a process.
AOPs rely on the production of highly reactive hydroxyl radicals (OH) in situ. These reactive species are the most powerful oxidants that can be used in water, and they can oxidize almost any compound present in the water matrix, often at a diffusion-controlled reaction speed. As a result, once formed, OH reacts in an unselective manner, and contaminants are quickly and efficiently fragmented and converted into small inorganic molecules.
One or more primary oxidants (e.g., ozone, hydrogen peroxide, oxygen) and/or energy sources (e.g., ultraviolet light) or catalysts are used to produce hydroxyl radicals (e.g. titanium dioxide). To achieve the highest OH yield, precise, pre-programmed dosages, sequences, and combinations of these reagents are used. In general, when used properly, AOPs can reduce contaminant concentrations from several hundred ppm to less than 5 ppb, significantly lowering COD and TOC, earning it the title of "water treatment processes of the future."
What are the benefits of photo oxidation?
Photo-oxidation for contaminant destruction has a number of advantages that only a few technologies have:
· Toxic pollutants are eliminated by converting them into inert substances (water, CO2 and mineral salts).
· The process is non-selective and can decompose almost any organic molecule, even complex ones.
· There is no need for any additional pre- or post-treatment procedures.
· The process consumes very little energy because it takes place at moderate temperatures (30-80 °C) and the radiation source could be solar energy.
· The chemicals used are inexpensive and widely available.
Given these benefits, photo-oxidation is an important effluent and process water treatment technique for a variety of industries, including the chemical, food, pharmaceutical, textile, and electroplating industries, among others, for removing species such as cyanide, Zn, Ni, antibiotics, hormones, organochlorides, organic polyphosphates, and nitrogenous and aromatic organic and heteroaromatic compounds.