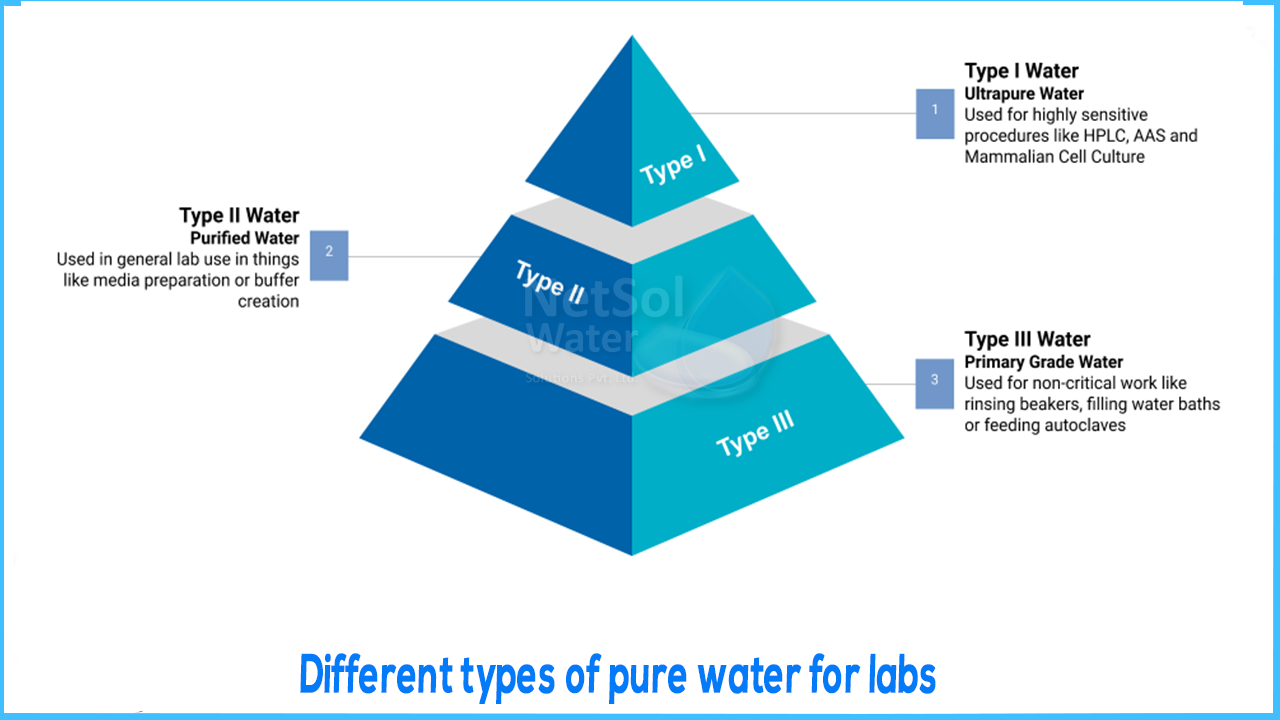
Different types of pure water for labs
- 1. FEED WATER: Feed water is also known as raw or potable water, and its quality is determined by its source. While deep water is naturally filtered by layers of rock and soil, water from surface sources such as lakes and reservoirs are susceptible to pollution. Dissolved ions, minerals, microorganisms, and organic compounds are among the most significant contaminants in feed water.
The color, odour, and turbidity of raw or feed water are typically measured. One can indeed look for chemical properties such as pH and hardness, as well as bacteriological properties.Feed water should always be tested and appropriate pre-treatment measures should be implemented to ensure that the quality is sufficient to not harm downstream purification technology.
Depth-filters are the most common type of pre-treatment. This method involves passing raw water through a series of winding fibres that attract and trap impurities. Carbon is also used to bind chlorine ions, which cause rapid deterioration of reverse osmosis (RO) membranes if not removed. Another prevalent step is to install a water softener, which reduces the hardness level of the water before it reaches the RO membrane. Hard water causes scaling of the RO membrane and shortens its lifespan.
- 2. GENERAL LABORATORY GRADE WATER:General laboratory grade water, also known as Type 2, is created by combining reverse osmosis with another technology such as ion exchange or electrical ion exchange (EDI). Deionization, also known as ion exchange, is the process of removing ions from RO water using synthetic resins. As the water passes through the ion exchange beads, chemical reactions take place, resulting in the removal of ions. This process is repeated until all unwanted ions are replaced by hydrogen and hydroxyl ions, which when combined form pure water.
EDI is a form of active purification that combines electrodialysis and ion exchange. Within an EDI cell, water is then passed between an anion permeable membrane and a cation permeable membrane. Ion exchange resin is loosely packed in the cell chamber. Ions are then fascinated to the oppositely charged electrode, but they are flushed away before they can reach it, removing them from the water.
These two processes combine to produce Type 2 water, which has a resistivity of 1-15M-cm and is suitable for general applications such as buffer and media production, general chemistry, and spectrophotometry.
- 3. ULTRAPURE WATER: Ultrapure (Type 1) water, with a resistivity of 18.2 M-cm at 25°C, is required for diagnostic laboratories. Type 1 water is commonly used in flow cytometry, pyrogen sensitive applications, and cell and tissue culture.Water with such a type of resistivity may still contain organic contaminants, endotoxins, and nucleases that have no effect on resistivity values, necessitating the use of other technologies to remove them.
Bacteria and organic levels are kept to a minimum by using a dual wavelength ultraviolet (UV) light (185nm and 254nm). The water goes through a vessel containing a UV light, and the light harm any genetic molecules required for reproductive functions as it passes through. This harm prevents the microorganism from multiplying or replicating, resulting in no infection.
DNase/RNase-free water can also be produced using an ultrafilter (UF). Filtration process removes particles and macromolecules by using size exclusion and is sometimes charged to attract contaminants. This innovation is used at the system's end to ensure the near-complete removal of macromolecular impurities.
- 4. PRIMARY GRADE WATER: The most cost-effective way to reduce water contaminants is to use primary grade pure water, which uses carbon filtration and RO technology.
RO removes up to 99% of contaminants from feed water by passing water through a semipermeable membrane from a less concentrated solution to a more concentrated solution. The osmotic flow is reversed by applying external pressure to the more concentrated side, which forces the water through the membrane and deposits the impurities on the surface. RO technology uses diffusion rather than separation to reject particles with a higher molecular weight. The temperature of the feed water, the pressure, and the physical condition of the RO membrane are all factors that influence rejection rates. As a result, while rejection rates vary, they tend to rise as the ionic charge and size of the molecule increase. As a result, RO water cannot be classified specifically.