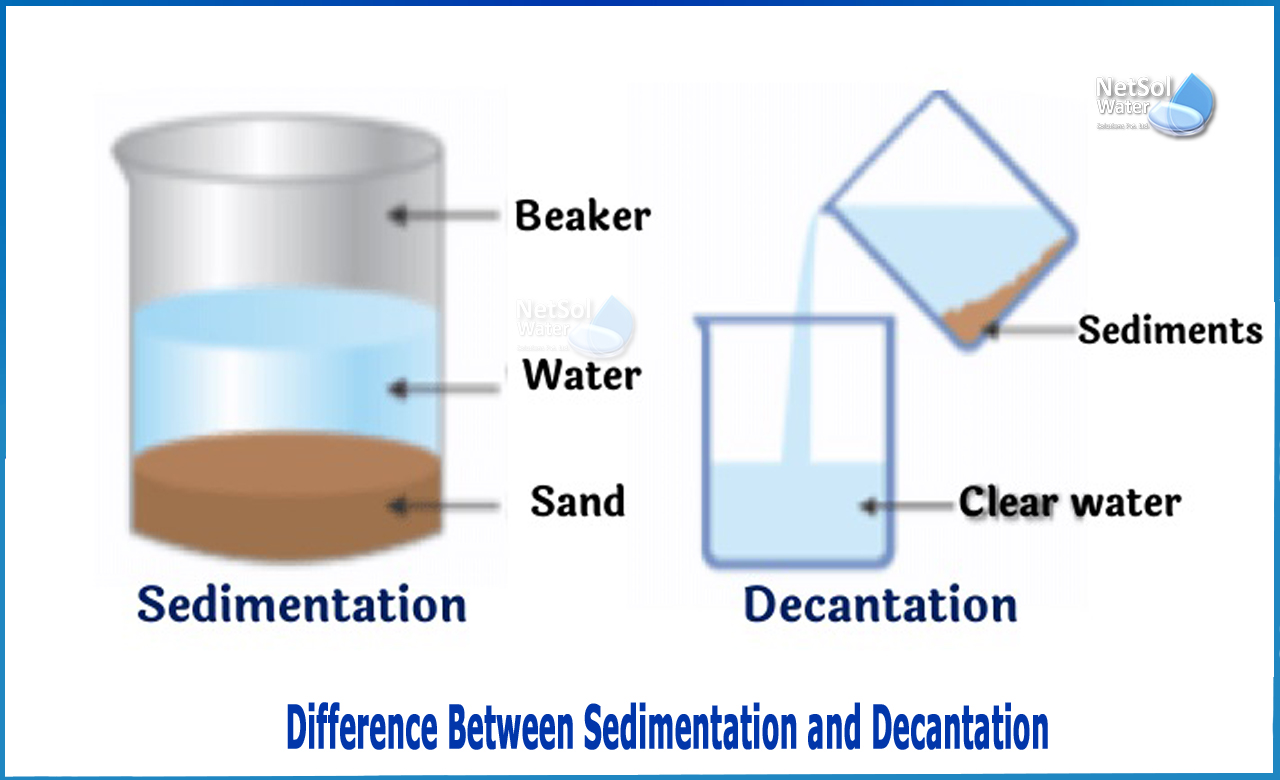
What is the Difference between Sedimentation and Decantation?
The main distinction between sedimentation and decantation is that sedimentation enables two compound to be separated by settling one, whereas decantation allows two substances to be separated by pouring off one.
In analytical chemistry, both sedimentation and decantation are significant separation processes. These methods can be used to separate two immiscible compounds.
Sedimentation happens when sediment forms naturally owing to gravity or centrifugal acceleration, whereas decantation is a process that we use to separate two substances. Furthermore, decantation can be used in the separation of silt from its fluid.
What is sedimentation?
The process of settling or being deposited as a sediment is known as sedimentation. The propensity of particles in a suspension to settle out of the fluid is what we might call it. This occurs as a result of the particles' reaction to their mobility through the fluid.
Gravity, centrifugal acceleration, or electromagnetism are all forces that operate on these particles. We may pour out the liquid above the silt and thereby separate the sediment from the fluid when the heavier particles fall on the bottom of the fluid.
We can use sedimentation in water/wastewater treatment facilities to remove undesirable particles from dirty water.
What is decantation, and how does it work?
Decantation is the process of pouring off one component to separate two immiscible compounds. This method can be used with two immiscible liquids or a liquid-solid combination (a suspension). If the combination of the two immiscible liquids to be separated is in a container, the less dense liquid layer (on top of the container) may easily be poured out. This allows the less dense liquid to be separated from the denser liquid. However, in the majority of cases, this separation is insufficient. Furthermore, this approach may be used to separate a solid from a liquid. By pouring out the fluid, we may separate silt from its fluid.
This method may also be used to separate two non-mixing liquids, such as oil and water. When we remove the oil and water combination from the container, two distinct layers appear, with water at the bottom and oil, which is lighter, at the top. The oil layer on top may be removed by pouring it into another vessel, leaving the water layer at the bottom.
Difference Between Decantation and Sedimentation
Decantation is the act of separating two immiscible substances by pouring off one component, whereas sedimentation is the process of settling or being deposited as sediment. This is the primary distinction.
Another distinction between sedimentation and decantation is the method they use. The sedimentation process separates the particles of the mixture using gravity, centrifugal acceleration, or electromagnetism, but the decantation process does not require any force acting on the particles of the mixture.
Conclusion
Separation techniques such as sedimentation and decantation can be used to separate two immiscible substances such as solids from liquids or liquids from liquids.
What can we offer?
If you need help designing an efficient wastewater treatment system, contact Netsol Water. We can help you with design calculations, budgetary expenses, preliminary layouts, and a lifetime cost analysis.
Netsol Water is Greater Noida-based leading water & wastewater treatment plant manufacturer. We are industry's most demanding company based on client review and work quality. We are known as best commercial RO plant manufacturers, industrial RO plant manufacturer, sewage treatment plant manufacturer, Water Softener Plant Manufacturers and effluent treatment plant manufacturers. Apart from this 24x7 customer support is our USP. Call on +91-9650608473, or write us at enquiry@netsolwater.com for any support, inquiry or product-purchase related query.