What is the definition of water demineralization?
Demineralization is a method of purifying water. While the term demineralization can refer to any treatment technique that removes minerals from water, it is usually reserved for ion exchange (IX) processes that remove ionic mineral pollutants almost completely. The phrases deionization and demineralization are frequently used interchangeably.
What is the process of demineralization?
Demineralization is the removal of dissolved mineral solids using an IX (Ion-Exchange) process. But, before we delve into the mechanics of demineralization, let's review the fundamentals of an IX reaction.
Minerals and salts dissociate into their constituent ions in the presence of water. These dissolved solids are made up of anions (negatively charged ions) and cations (positively charged ions), both of which are attracted to counterions (or ions of an opposing charge). An IX column contains a resin made consisting of plastic beads with an ionic functional group attached to them.
Through mutual electrostatic attraction, these functional groups loosely retain ions with opposing charges. Water containing dissolved ions is added to the resin during an active IX cycle.Even as the resultant solution is drained away, the ions in solution will trade places with the ions on the resin beads, clinging to the resin's functional groups. When one ion has a stronger affinity for the functional group than the one already present, IX occurs.
The presence of certain ionic pollutants will determine whether anionic or cationic resins are required. The exchange of ions in a normal IX reaction merely results in the replacement of contaminated ions with less undesirable ions. The goal of an IX sodium softening system, for example, is to replace hardness ions (e.g., Ca2+ or Mg2+) in solution with sodium ions (Na+).As a result, the treated solution will be soft to the touch, with a higher sodium ion concentration.

While this is appropriate in many cases, other operations require near-total dissolved solids removal. This is when demineralization enters the picture. Cations in the feed water are exchanged for hydrogen (H+) ions and anions for hydroxyl (OH–) ions during demineralization.
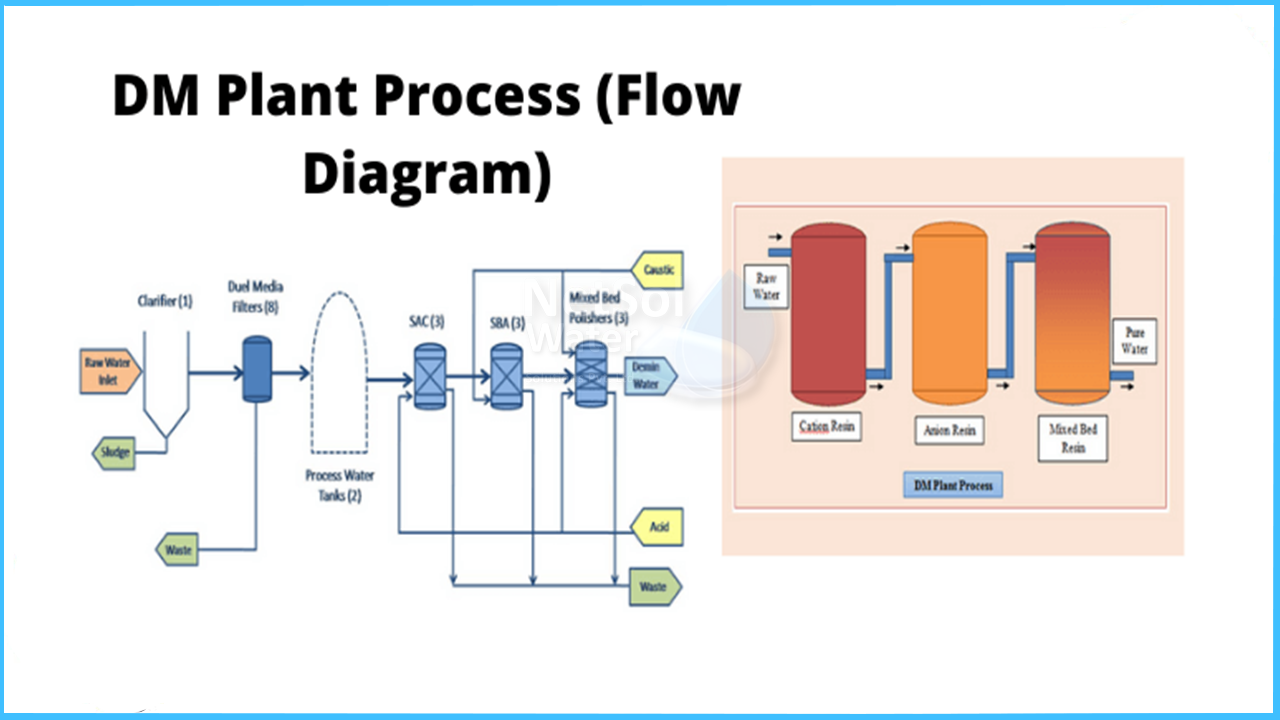
Demineralized water treatment plant working process
- 1. Two-bed IX
Two-bed or dual-bed exchangers treat a stream with two or more IX resin beds or columns, each carrying a different type of IX resin. A stream is initially treated with a strong acid cation (SAC) resin, which collects dissolved cations and releases hydrogen (H+) ions in exchange in two-bed demineralization.
The mineral acid solution is subsequently sent to the resin bed for strong base anion (SBA). The anionic pollutants are sequestered in this second stage, which also releases hydroxide (OH–) ions, which combine with the hydrogen ions (H+) to produce water.
The resulting stream has a pH of almost neutral and is low in TDS.While two-bed exchangers are successful at demineralization, salt leakage can degrade the output quality, particularly in streams with high TDS and/or low pH.
- 2. Mixed-bed IX
When compared to twin-bed systems, mixed-bed exchangers provide better water quality. Mixed-bed ion exchangers contain a variety of IX resins within a single IX column. When a stream is fed to the unit, the cation and anion exchange reactions occur simultaneously within the unit, solving salt leakage difficulties that can degrade the water quality generated by a twin-bed IX system.
While mixed-bed exchangers produce better water, they also necessitate a more time-consuming resin renewal procedure.Mixed-bed units are also more sensitive to resin fouling ands poor system performance due to changes in stream contents, hence they're usually utilized after other treatment methods.