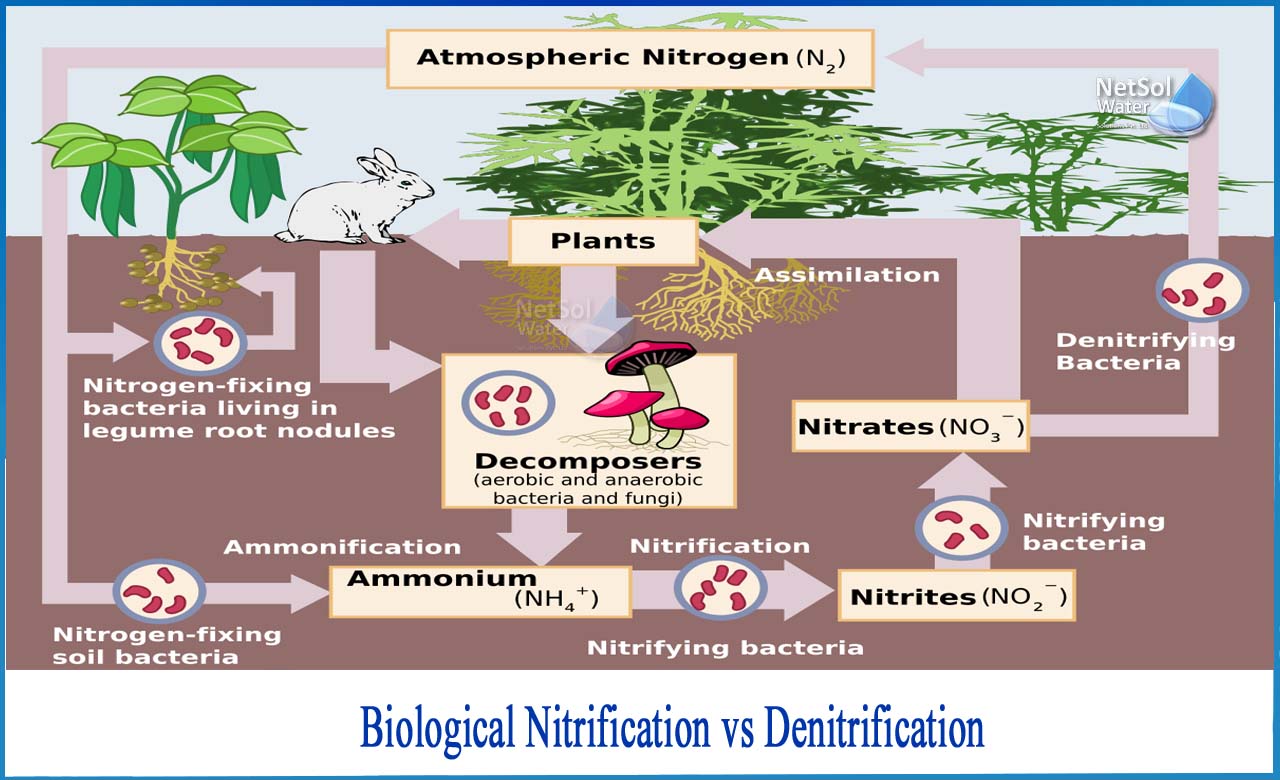
What Is the Difference Between Nitrification and Denitrification?
Bacteria uses a two-step biological process to remove nitrogen from wastewater:
Nitrification and denitrification.
Technically, ammonification comes first, followed by nitrification and denitrification. The bulk of the nitrogen in raw sewage (urea and faecal debris) is transformed from organic-nitrogen to ammonia during its journey through sewer pipes, a process known as hydrolysis.
The microbe-mediated process of oxidizing ammonia to remove nitrogenous chemicals from wastewaters is known as biological nitrification. Ammonia nitrogen concentrations in domestic sewage range from 20 to 40 mg/L. (NH4-N). Biodegradation of nitrogen-containing organic materials, such as protein and nucleic acid, results in the release of ammonia. The release of this ammonia into receiving streams is directly hazardous to fish and other species, as well as depletes oxygen levels.
What is Nitrification?
Ammonia removal is becoming increasingly stringent in permit requirements, making it one of the most crucial and complex processes in wastewater treatment facilities to manage. Environmental conditions, shocks, toxicity, and solids loss can all affect ammonia levels.
Nitrification is a crucial step in biological water purification because it removes nitrogen from wastewater.In waste water, nitrogen is generally found as ammonium (NH4+) or bound in organic molecules. When microbes deplete these molecules, organically bound nitrogen is converted to ammonium.Aerobic bacteria oxidize ammonium to nitrate in a two-step biological process known as nitrification.The transformation of ammonium into nitrate (nitrification) is accomplished by maintaining two types of bacteria, Nitrosomonas and Nitrobacter.
Nitrosomonas provides the first step, the transformation of ammonium to nitrite (NO2).
Nitrobacter provides the second step, the transformation of nitrite to nitrate (NO3-)
Both types of nitrifiers are autotrophs, which mean that they build cellular materials using carbon dioxide or carbonate and obtain energy from the chemical conversion of ammonia into nitrite and nitrate. When compared to more typical heterotrophic (need organic carbon for development) wastewater bacteria, autotrophic nitrifying bacteria get less energy throughout their metabolic operations. This lower energy level results in retarded cellular growth. In the case of Nitrosomonas, this is especially true. Nitrosomonas are the bacteria that convert ammonia to nitrite. Nitrobacter's development, on the other hand, is hampered since they are responsible for converting nitrite to nitrate.
Step 1: NH4+ + 3/2 O2 NO2- + 2H++ H2O (Nitrite)
Step 2: NO2- + 1/2 O2 N03-(Nitrate)
The final equation of the conversion of ammonium to nitrate is:
NH4 + 2O2![]() NO3 + 2H + H2O (water).
NO3 + 2H + H2O (water).
For nitrifying bacteria to grow well, a number of conditions must be met:
a) Removal of BOD/COD > 2 mg/L (D.O).
b) The ideal temperature is between 85 and 95oF (30 and 35oCelsius.) They can, however, develop in temperatures ranging from 50 to 100oF (10 to 38 oC). Little nitrification can be predicted at temperatures below 41°F (5°C) and over 115°F (45°C).
c) pH range: 7.2-8.0.
d) Many H+ ions are released during the conversion of ammonia to nitrate, resulting in a pH reduction. A supply of alkalinity is necessary to maintain a constant pH. For each milligram of ammonia eliminated, biological ammonia oxidation needs 7.1 mg of alkalinity in the form of CaCO3. As a result, 7 ppm alkalinity is required for every 1 ppm NH in the influent.
e) MCRT is greater than ten days.
What is Denitrification?
Nitrification may not be adequate in some applications, such as discharge of effluent into confined water bodies or recycling to water sources. When it comes to nitrogen removal, one option is to follow biological nitrification with denitrification.
Following nitrification in a biological water treatment, denitrification is usually the next stage. Nitrate (NO3) and nitrite (NO2) are converted to nitrogen here (N2). The nitrogen gas escapes from the water and into the atmosphere. Because air is made up of 78 percent nitrogen (N2) and 21% oxygen (O2), N2 does not pollute the environment in any way.
Denitrification may be accomplished by a huge number of aerobic microorganisms. These bacteria use nitrate and nitrite as oxygen sources when there isn't any oxygen in the water.Denitrification occurs when oxygen levels are depletedand nitrate becomes the primary source for microorganisms.
Nitrate, NO3- - Nitrite, NO2-- Nitric oxide, NO - Nitrous oxide, N2O - Nitrogen, N2
How can Netsol Water help you in this?
Netsol Water is one of the leading water and wastewater treatment company in India offering projects and services in the field of water and wastewater treatment plant manufacturing and supplying machines like compact sewage treatment plant, and much morewhich not only turns your wastewater into usable water but also acts as a savior of Mother earth and its precious resource “water”.
Netsol Water is Greater Noida-based leading water & wastewater treatment plant manufacturer. We are industry's most demanding company based on client review and work quality. We are known as best commercial RO plant manufacturers, industrial RO plant manufacturer, sewage treatment plant manufacturer, Water Softener Plant Manufacturers and effluent treatment plant manufacturers. Apart from this 24x7 customer support is our USP. Call on +91-9650608473, or write us at enquiry@netsolwater.com for any support, inquiry or product-purchase related query.