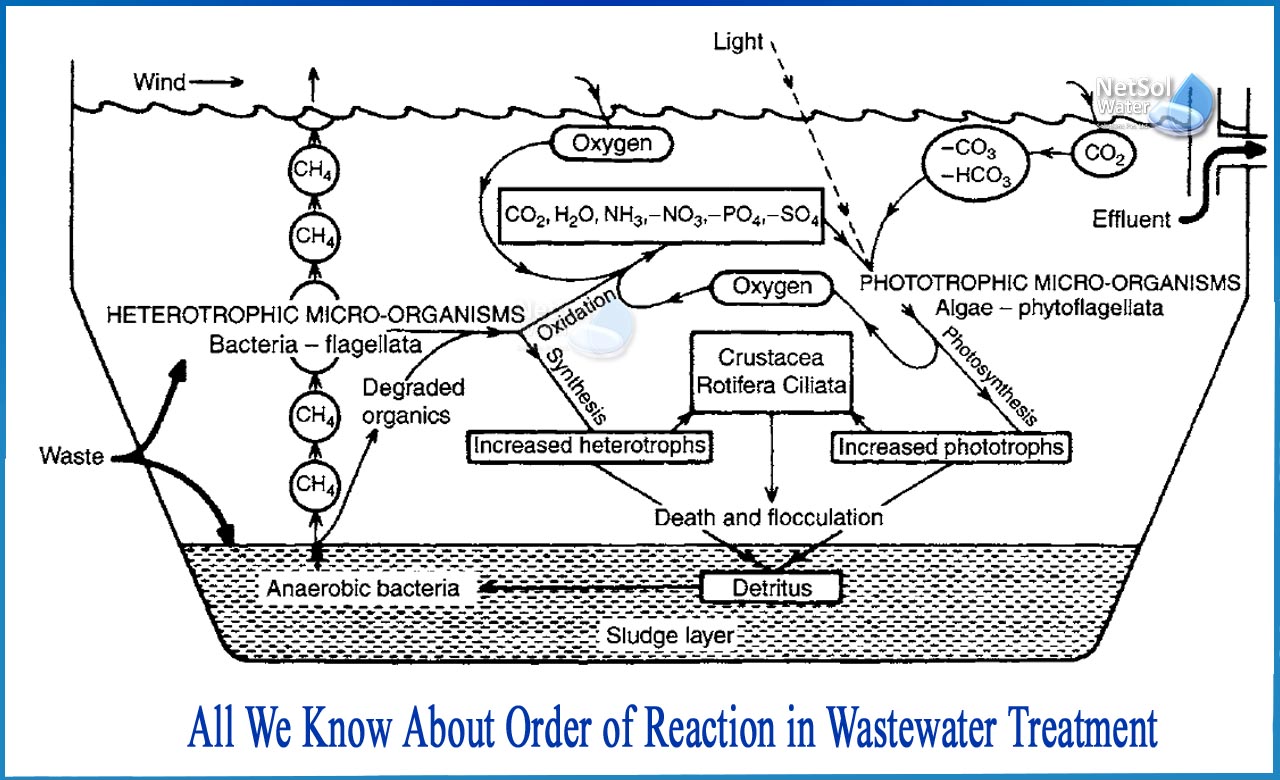
What is the order of reaction in Wastewater treatment?
Chemical or biochemical reaction kinetics is the study of chemical or biochemical reactions in terms of reaction rates, the effects of conditions under the reaction, molecular rearrangements, intermediate formation, and catalytic involvement.
The word dynamics is derived from the Greek word kinesis and means movement. Therefore, the reaction rate of a chemical or biochemical reaction is primarily related to the reaction rate and everything else that affects it.
Reaction rate depends on the concentration of the reactants!
In general, the reaction rate depends on the concentration of the reactants. It may also depend on the concentration of other species not included in the stoichiometric formula.
The dependence of the reaction rate on the reactant concentration can be expressed mathematically with respect to the reaction rate constant and the power of the reactant concentration.
For a general reaction format,
r = kCA or v?=k [A]x[B]y
Where,“k” is the reaction rate constant and “CA” and “CB” are the concentrations of reactants A and B.
And, “v?”isreactionrate, “x” is order of reaction with respect to A and “y” is the order of reaction with respect to B
Reactions are generally categorized as 0th, 1st, 2nd, or mixed (higher) reactions based on the value of (a + b).
Types of Reaction
1: Zero order reaction - The speed of the zero-order reaction (order = 0) is constant. This rate is independent of the concentration of the reactants.
Rate of equation is r = k
2: First Order reaction - The first-order reaction (order = 1) has a rate proportional to the concentration of one of the reactants. A well-known example of a primary reaction is the phenomenon of radioactive decay.
Rate equation is r = kCA (or CB instead of CA)
3: Third order reaction - The rate of a second-order (order = 2) reaction is proportional to the square of the concentration of a single reactant or the product of the concentrations of two reactants.
Rate of equation = kCA2 (or replace A with B)
4: Mixed or higher-order reaction - Mixed-order reaction, like some biochemical reactions, has a fractional order of its rate.
Rate of equation = kCA 1/3
Conclusion
These order of reactions are very much necessary to determine the rate of reactions in order to successfully operate the treatment plant.
We at Netsol Water have more than 10 years of experience in waste water treatment plant manufacturing and operations. For water, wastewater or sludge treatment, we offer a complete spectrum of wastewater treatment products. Our engineers would gladly collaborate with your team to create the right design that meets your budget and objectives.
Netsol Water is Greater Noida-based leading water & wastewater treatment plant manufacturer. We are industry's most demanding company based on client review and work quality. We are known as best commercial RO plant manufacturers, industrial RO plant manufacturer, sewage treatment plant manufacturer, Water Softener Plant Manufacturers and effluent treatment plant manufacturers. Apart from this 24x7 customer support is our USP. Call on +91-9650608473, or write us at enquiry@netsolwater.com for any support, inquiry or product-purchase related query.